Influence of Sexual Dimorphism on Stable Isotopes and Trace Element Concentrations in the Greater Hooked Squid Moroteuthopsis Ingens from New Zealand Waters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Southern Ocean Squids Using Nets and Beaks
Marine Biodiversity (2020) 50:98 https://doi.org/10.1007/s12526-020-01113-4 REVIEW A review of Southern Ocean squids using nets and beaks Yves Cherel1 Received: 31 May 2020 /Revised: 31 August 2020 /Accepted: 3 September 2020 # Senckenberg Gesellschaft für Naturforschung 2020 Abstract This review presents an innovative approach to investigate the teuthofauna from the Southern Ocean by combining two com- plementary data sets, the literature on cephalopod taxonomy and biogeography, together with predator dietary investigations. Sixty squids were recorded south of the Subtropical Front, including one circumpolar Antarctic (Psychroteuthis glacialis Thiele, 1920), 13 circumpolar Southern Ocean, 20 circumpolar subantarctic, eight regional subantarctic, and 12 occasional subantarctic species. A critical evaluation removed five species from the list, and one species has an unknown taxonomic status. The 42 Southern Ocean squids belong to three large taxonomic units, bathyteuthoids (n = 1 species), myopsids (n =1),andoegopsids (n = 40). A high level of endemism (21 species, 50%, all oegopsids) characterizes the Southern Ocean teuthofauna. Seventeen families of oegopsids are represented, with three dominating families, onychoteuthids (seven species, five endemics), ommastrephids (six species, three endemics), and cranchiids (five species, three endemics). Recent improvements in beak identification and taxonomy allowed making new correspondence between beak and species names, such as Galiteuthis suhmi (Hoyle 1886), Liguriella podophtalma Issel, 1908, and the recently described Taonius notalia Evans, in prep. Gonatus phoebetriae beaks were synonymized with those of Gonatopsis octopedatus Sasaki, 1920, thus increasing significantly the number of records and detailing the circumpolar distribution of this rarely caught Southern Ocean squid. The review extends considerably the number of species, including endemics, recorded from the Southern Ocean, but it also highlights that the corresponding species to two well-described beaks (Moroteuthopsis sp. -

Technical Report No. 447 1974 •
FISHERIES RESEARCH BOARD OF CANADA TECHNICAL REPORT NO. 447 1974 • ... FISHERIES RESEARCH BOARD OF CANADA Technical Reports FRB Technical Reports are research documents that are of sufficient importance to be preserved, but which ·for some reason are not appropriate for primary scientific publication. No restriction is placed on subject matter and the series should reflect the broad research interests of FRB. These Reports can be cited in publications, but care should be taken to indicate their manuscript status. Some of the material in these Reports will eventually appear in the primary scientific literature. Inquiries concerning any particular Report should be directed to the issuing FRB establishment which is indicated on the title page. FISHERIES AND MARINE SERVICE TECHNICAL REPORT NO. 44 7 THE SQUID OF BRITISH COLUMBIA AS A POTENTIAL FISHERY RESOURCE - A PRELIMINARY REPORT by S.A. Macfarlane and M. Yamamoto Fisheries and Marine Service Vancouver Laboratory Vancouver, B.C. TABLE OF CONTENTS Page No. I. INTRODUCTION 1 II. BIOLOGICAL ASPECTS 3 III. COMMERCIAL ASPECTS 8 A. Fishing Methods 8 B. International Squid Fisher,y 15 c. Status of Squid in British Co1uabia 19 IV. NUTRITIONAL ASPECTS 27 v. PROCESSING 28 VI. DISCUSSION 30 VII. ACKNOWLEDGMENTS 32 VIII. REFERENCES 33 1. I. INTRODUCTION Available catch statistics from 1965 through 1971 indicate • that world-wide landings of squid totalled roughly 700,000 metric tons annually. An additional 100,000 metric tons of cuttlefish and about 160,000 metric tons of octopus were also landed annually. Apart from the well-established squid fishery in the Monterey area of California and the relatively minor inshore squid fishery off Newfoundland, the North American fishing industry has tended to ignore the possibility of further exploitation and utilization of this resource. -

Vertical Distribution of Pelagic Cephalopods *
* Vertical Distribution of Pelagic Cephalopods CLYDE F. E. ROPER and RICHARD E. YOUNG SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 209 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Insti- tution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, com- mencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. These pub- lications are distributed by mailing lists to libraries, laboratories, and other interested institutions and specialists throughout the world. Individual copies may be obtained from the Smithsonian Institution Press as long as stocks are available. S. DILLON RIPLEY Secretary Smithsonian Institution SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 209 Vertical Distribution of Pelagic Cephalopds Clyde F. -

A Review of Direct and Indirect Impacts of Marine Dredging Activities on Marine Mammals
A review of direct and indirect impacts of marine dredging activities on marine mammals Family Scientific name Common name Range of best Frequency of Minimum Methodology Diet Region Habitat Documented Effects of Potential Effects of Dredging (excluding (including hearing (10 dB minimum hearing Dredging subspecies) subspecies) from max; kHz) hearing threshold (dB threshold (kHz) re 1 µPa) Otariidae Arctocephalus Cape & Unknown; — — — Fish (e.g. Emmelichthys nitidus, F, J (Kirkman et Continental shelf waters (IUCN, — Habitat destruction, increase in pusillus Australian fur fundamental Pseudophycis bachus, Trachurus al., 2007; IUCN, 2012) turbidity, changes to prey seal frequency of declivis, Neoplatycephalus 2013; Perrin, availability, masking, incidental male in air barks Richardsoni) (Australian fur seal) 2013) capture or injury, avoidance & is 0.14 & female (Page et al., 2005) an increase in shipping traffic in air barks is 0.15 (Tripovich et al., 2008) Arctophoca Antarctic fur seal Unknown; peak — — — Fish (e.g. Gymnoscopelus A, F, J (IUCN, Forage in deep waters (>500 m) — Habitat destruction, increase in gazella frequency of in piabilis, Electrona subaspera, 2013; Perrin, with a strong chlorophyll turbidity, changes to prey air barks is 0.3– Champsocephalus gunnari) 2013; Reeves et concentration & steep availability, masking, incidental 5.9 (Page et al., (Guinet et al., 2001) al., 2002) bathymetric gradients, otherwise capture or injury, avoidance & 2002) remains close to the colony in an increase in shipping traffic areas with Polar -

Squids of the Family Onychoteuthidae Gray, 1847 in the Southeastern Pacific Ocean
Lat. Am. J. Aquat. Res., 44(2): 416-421, 2016 Onychoteuthid squids from Chile 416 1 DOI: 10.3856/vol44-issue2-fulltext-23 Short Communication Squids of the family Onychoteuthidae Gray, 1847 in the southeastern Pacific Ocean 1 1 Christian M. Ibáñez & Alina F. Cifuentes-Bustamante 1Departamento de Ecología y Biodiversidad, Facultad de Ecología y Recursos Naturales Universidad Andres Bello, Santiago, Chile Corresponding author: Christian M. Ibáñez ([email protected]) ABSTRACT. Hooked squids (Family Onychoteuthidae Gray, 1847) inhabit all oceans of the world except the Arctic. This family is currently comprised of 25 species belonging to seven genera. In the southeastern Pacific Ocean, approximately five onychoteuthid species have been previously identified, but true identity of these taxa is uncertain. We reviewed museum collections, from Chile, United States and New Zealand, and literature to elucidate the presence of hooked squids in the southeastern Pacific Ocean. The present status of the Onychoteuthidae from the southeastern Pacific only includes four species: Onychoteuthis aequimanus, Onykia ingens, Onykia robsoni, and Kondakovia nigmatullini. Keywords: Onychoteuthidae, Onychoteuthis, Onykia, Kondakovia, hooked squids, Chile. Calamares de la familia Onychoteuthidae Gray, 1847 en el Océano Pacífico suroriental RESUMEN. Los calamares ganchudos (Familia Onychoteuthidae Gray, 1847) habitan en todos los océanos excepto en el Ártico. Esta familia está compuesta actualmente de 25 especies pertenecientes a siete géneros. En el Océano Pacífico suroriental, aproximadamente cinco especies de Onychoteuthidae han sido identificadas previamente, pero su estatus taxonómico es incierto. Se revisaron las colecciones de museos de Chile, Estados Unidos y Nueva Zelanda, y la literatura para dilucidar la presencia de calamares con ganchos en el Pacífico suroriental. -

Cephalopod Beak Sections Used to Trace
Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study José Queirós, Paco Bustamante, Yves Cherel, Joao Coelho, José Seco, Jim Roberts, Eduarda Pereira, José Xavier To cite this version: José Queirós, Paco Bustamante, Yves Cherel, Joao Coelho, José Seco, et al.. Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study. Marine Environmental Research, Elsevier, 2020, 161, pp.105049. 10.1016/j.marenvres.2020.105049. hal-02904190 HAL Id: hal-02904190 https://hal.archives-ouvertes.fr/hal-02904190 Submitted on 21 Jul 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study José P. Queirós*a, Paco Bustamanteb,c, Yves Chereld, João P. Coelhoe, José Secof,g, Jim Robertsh, Eduarda Pereiraf & José C. Xaviera,i a – University of Coimbra, MARE – Marine and Environmental Sciences Centre, Department of Life Sciences, 3000-456, Coimbra, Portugal b - Littoral Environnement et Sociétés (LIENSs), UMR 7266 CNRS-La Rochelle Université, 2 rue Olympe de Gouges, 17000 La Rochelle, France c - Institut Universitaire de France (IUF), 1 rue Descartes 75005 Paris, France d - Centre d’Etudes Biologiques de Chizé, UMR 7372 du CNRS–La Rochelle Université, 79360 Villiers–en–Bois, France. -

University of Groningen Sperm Storage and Mating in the Deep-Sea
University of Groningen Sperm storage and mating in the deep-sea squid Taningia danae Joubin, 1931 (Oegopsida Hoving, Hendrik Jan T.; Lipinski, Marek R.; Videler, John J.; Bolstad, Kat S. R. Published in: Marine Biology DOI: 10.1007/s00227-009-1326-7 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2010 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Hoving, H. J. T., Lipinski, M. R., Videler, J. J., & Bolstad, K. S. R. (2010). Sperm storage and mating in the deep-sea squid Taningia danae Joubin, 1931 (Oegopsida: Octopoteuthidae). Marine Biology, 157(2), 393- 400. https://doi.org/10.1007/s00227-009-1326-7 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Diverse Foraging Strategies by a Marine Top Predator Sperm Whales
Author’s Accepted Manuscript Diverse foraging strategies by a marine top predator: sperm whales exploit pelagic and demersal habitats in the Kaikōura submarine canyon M. Guerra, L. Hickmott, J. van der Hoop, W. Rayment, E. Leunissen, E. Slooten, M. Moore www.elsevier.com PII: S0967-0637(17)30121-8 DOI: http://dx.doi.org/10.1016/j.dsr.2017.08.012 Reference: DSRI2828 To appear in: Deep-Sea Research Part I Cite this article as: M. Guerra, L. Hickmott, J. van der Hoop, W. Rayment, E. Leunissen, E. Slooten and M. Moore, Diverse foraging strategies by a marine top predator: sperm whales exploit pelagic and demersal habitats in the Kaikōura submarine canyon, Deep-Sea Research Part I, http://dx.doi.org/10.1016/j.dsr.2017.08.012 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Diverse foraging strategies by a marine top predator: sperm whales exploit pelagic and demersal habitats in the Kaikōura submarine canyon Guerra M.1*, Hickmott L.2,3, van der Hoop J.4, Rayment W.1, Leunissen E.1, Slooten E.1, Moore M.5 1 University of Otago, Dunedin, New Zealand 2 Scottish Oceans Institute, University of St Andrews, St Andrews, UK 3 Open Ocean Consulting, Petersfield, Hants, UK 4 Aarhus University, Aarhus, Denmark 5 Woods Hole Oceanographic Institution, Woods Hole, USA * Corresponding author – [email protected] Running page head: Foraging behaviour of sperm whales Key words: submarine canyon; sperm whale; foraging; Kaikoura; echolocation; demersal ABSTRACT The submarine canyon off Kaikōura (New Zealand) is an extremely productive deep-sea habitat, and an important foraging ground for male sperm whales (Physeter macrocephalus). -

Fao Species Identification Sheets for Fishery Purposes
3D FAO SPECIES IDENTIFICATION SHEETS FOR FISHERY PURPOSES WESTERN CENTRAL ATLANTIC (Fishing Area 31) edited by W. Fischer Marine Resources Service Fishery Resources and Environment Division FAO Fisheries Department This publication has been prepared and printed with the support of the UNDP/FAO International Project for the Development of Fisheries in the Western Central Atlantic VOLUME VI CONTENTS: Lobsters Shrimps and Prawns True Crabs Stomatopods Bivalves Gastropods Chitons * Cephalopoda + Sea Turtles FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1978 Bibliographic Reference: Roper, C.F.E. In: Fi.ch«, w. CEO cms) Rome, FAO, pag.var. FAO species identification sheets for fishery purposes. Western Central Atlantic (fishing area 31>. Vols. 1-7 Identification sheets. Taxonomy. Geographic distribution. Fisheries. Vernacular names. Bony fishes. Sharks. Batoid fishes. Lobsters. Shrimps. True crabs. S tomato pods. Molluscs. Sea turtles. ASH. FAO Sheets Fishing Area 3 I TECHNICAL TERMS lamellae I hectocotylus (or modified •< portion) suckers buccal membrane funnel groove oegopsid eye normal sucker: funne I funnel-mantle fusion funnel locking cartilage mantle locking cartilage 1 dactylus ~T"Y fin (posteriorly concave) _ fin (posteriorly V t convex) loss t— fin length example of hectocotylized arm in male (Illex ittecebrosue) __cail arm I (dorsal) mantle length buccal lappet J a composite diagram illustrating beak jaws basic squid (teuthoid) features ventral view buccal suckers buccal connective (ventrally attached) buccal connective (dorsally attached arm IV (ventral) diagram of oral surface of brachial crown and buccal membrane oval with inward simple, -^-shaped J_-shaped subtriangular projecting knobs straight basic types of funnel locking cartilage (cartilaginous grooves that lock with corresponding ridges on inner mantle wall to keep base of funnel in position during water expulsion) - 2 - FAO Sheets CEFHALOPOOS Fishing Area 31 mantle Length lame 1Lae (internal) / funnel aperture suckers V" total length A. -
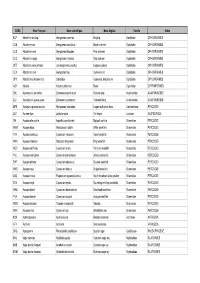
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa -

Redalyc.Squids of the Family Onychoteuthidae Gray, 1847 in The
Latin American Journal of Aquatic Research E-ISSN: 0718-560X [email protected] Pontificia Universidad Católica de Valparaíso Chile Ibáñez, Christian M.; Cifuentes-Bustamante, Alina F. Squids of the family Onychoteuthidae Gray, 1847 in the southeastern Pacific Ocean Latin American Journal of Aquatic Research, vol. 44, núm. 2, mayo, 2016, pp. 416-421 Pontificia Universidad Católica de Valparaíso Valparaíso, Chile Available in: http://www.redalyc.org/articulo.oa?id=175046298023 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Lat. Am. J. Aquat. Res., 44(2): 416-421, 2016 Onychoteuthid squids from Chile 416 1 DOI: 10.3856/vol44-issue2-fulltext-23 Short Communication Squids of the family Onychoteuthidae Gray, 1847 in the southeastern Pacific Ocean 1 1 Christian M. Ibáñez & Alina F. Cifuentes-Bustamante 1Departamento de Ecología y Biodiversidad, Facultad de Ecología y Recursos Naturales Universidad Andres Bello, Santiago, Chile Corresponding author: Christian M. Ibáñez ([email protected]) ABSTRACT. Hooked squids (Family Onychoteuthidae Gray, 1847) inhabit all oceans of the world except the Arctic. This family is currently comprised of 25 species belonging to seven genera. In the southeastern Pacific Ocean, approximately five onychoteuthid species have been previously identified, but true identity of these taxa is uncertain. We reviewed museum collections, from Chile, United States and New Zealand, and literature to elucidate the presence of hooked squids in the southeastern Pacific Ocean. The present status of the Onychoteuthidae from the southeastern Pacific only includes four species: Onychoteuthis aequimanus, Onykia ingens, Onykia robsoni, and Kondakovia nigmatullini.