Eastern Rockhopper Penguins Eudyptes Filholi As Biological
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Southern Ocean Squids Using Nets and Beaks
Marine Biodiversity (2020) 50:98 https://doi.org/10.1007/s12526-020-01113-4 REVIEW A review of Southern Ocean squids using nets and beaks Yves Cherel1 Received: 31 May 2020 /Revised: 31 August 2020 /Accepted: 3 September 2020 # Senckenberg Gesellschaft für Naturforschung 2020 Abstract This review presents an innovative approach to investigate the teuthofauna from the Southern Ocean by combining two com- plementary data sets, the literature on cephalopod taxonomy and biogeography, together with predator dietary investigations. Sixty squids were recorded south of the Subtropical Front, including one circumpolar Antarctic (Psychroteuthis glacialis Thiele, 1920), 13 circumpolar Southern Ocean, 20 circumpolar subantarctic, eight regional subantarctic, and 12 occasional subantarctic species. A critical evaluation removed five species from the list, and one species has an unknown taxonomic status. The 42 Southern Ocean squids belong to three large taxonomic units, bathyteuthoids (n = 1 species), myopsids (n =1),andoegopsids (n = 40). A high level of endemism (21 species, 50%, all oegopsids) characterizes the Southern Ocean teuthofauna. Seventeen families of oegopsids are represented, with three dominating families, onychoteuthids (seven species, five endemics), ommastrephids (six species, three endemics), and cranchiids (five species, three endemics). Recent improvements in beak identification and taxonomy allowed making new correspondence between beak and species names, such as Galiteuthis suhmi (Hoyle 1886), Liguriella podophtalma Issel, 1908, and the recently described Taonius notalia Evans, in prep. Gonatus phoebetriae beaks were synonymized with those of Gonatopsis octopedatus Sasaki, 1920, thus increasing significantly the number of records and detailing the circumpolar distribution of this rarely caught Southern Ocean squid. The review extends considerably the number of species, including endemics, recorded from the Southern Ocean, but it also highlights that the corresponding species to two well-described beaks (Moroteuthopsis sp. -

Forage Fish Management Plan
Oregon Forage Fish Management Plan November 19, 2016 Oregon Department of Fish and Wildlife Marine Resources Program 2040 SE Marine Science Drive Newport, OR 97365 (541) 867-4741 http://www.dfw.state.or.us/MRP/ Oregon Department of Fish & Wildlife 1 Table of Contents Executive Summary ....................................................................................................................................... 4 Introduction .................................................................................................................................................. 6 Purpose and Need ..................................................................................................................................... 6 Federal action to protect Forage Fish (2016)............................................................................................ 7 The Oregon Marine Fisheries Management Plan Framework .................................................................. 7 Relationship to Other State Policies ......................................................................................................... 7 Public Process Developing this Plan .......................................................................................................... 8 How this Document is Organized .............................................................................................................. 8 A. Resource Analysis .................................................................................................................................... -

Fishery Bulletin/U S Dept of Commerce National Oceanic
Abstract.- Gastrointestinal tract contents were evaluated from Prey selection by northern fur seals 73 female andjuvenile male north ern fur seals (Callorhinus ursinus) fCallorhinus ursinusJ in the eastern for analysis of their diet in the Bering Sea. Fur seals were col Bering Sea lected from August to October of 1981, 1982, and 1985. Juvenile Elizabeth Sinclair walleye pollock (Theragra chalco gramma) and gonatid squid were Thomas Loughlin the primary prey. Pacific herring National Marine Mammal Laboratory. Alaska Fisheries Science Center; (Clupea pallasi) and capelin National Marine Fisheries Service. NOM <Mallotus villosus), considered im 7600 Sand Point Way. N.E., Seattle, Washington 98115 portant fur seal prey in previous reports, were absent from the diet. Prey species and size varied William Pearcy among years and between near College of Oceanography, Oregon State University shore and pelagic sample loca Oceanography Administration Building 104 tions. Interannual variation in the Corvaflis, Oregon 9733 J importance ofpollock in the diet of fur seals was positively related to year-class strength of pollock. Midwater (n=23) and bottom (n=116) trawls were conducted at The Pribilof Island population (St. were conducted prior to the 1975 the location of fur seal collections George and St. Paul Islands) of 81 population decline and prior to to determine availability of fish northern fur seals (Callorhinus the 1970's development of a com and squid relative to prey species eaten by fur seals. The species and ursinus) represents approximately mercial walleye pollock (Theragra size composition of prey taken by 75% of the total species breeding chalcogramma) fishery in the fur seals was similar to midwater population. -

Full Text in Pdf Format
MARINE ECOLOGY PROGRESS SERIES Vol. 177: 115-131, 1999 Published February 11 Mar Ecol Prog Ser Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill J. P. Croxall*, K. Reid, P. A. Prince British Antarctic Survey, Natural Environment Research Council. High Cross, Madingley Road. Cambridge CB3 OET. United Kingdom ABSTRACT: Knowledge of relationships between prey availability and predator performance is the key to using predators as indicators of the state of marine systems and to assessing potential conse- quences of competition between natural predators and man for common resources. Fluctuations in the abundance of Antarctic knll are beiieved to have a substantial influence on the reproductive perfor- mance of krill-dependent top predator species in the Southern Ocean; few quantifications of such inter- actions exist. At South Georgia, for 2 years in which acoustic surveys revealed a major difference in knll abundance, we compared diet, prov~sioningof offspring and breeding success in 4 main predator species (2 penguins, 2 albatrosses, with supporting data from Antarctic fur seal) whose dependence on krd typically ranges from 20 to 90%. The 4-fold difference in krill biomass between 1986 (ca 30 g m-2) and 1994 (ca 7 g m-2)was accompanied by (1) an 88 to 90% reduction in the mass of krill in predator dlets (and some increase in the fish component), (2) greater prey diversity for most species, (3) reduced diet overlap between species and (4) a switch from krill to amphipods in macaroni penguin but no major dietary change in other species. -

Long-Term Fluctuations in Gonatid Squid (Gonatidae) Abundance in the Okhotsk Sea
Pacific Research Institute of Fisheries and Oceanography (TINRO-Centre) Long-term fluctuations in gonatid squid (Gonatidae) abundance in the Okhotsk Sea Mikhail A. Zuev Nikolai S. Vanin Oleg N. Katugin Gennady A. Shevtsov The main goal reveal annual distribution patterns of squids of the family Gonatidae in the Sea of Okhotsk basing on data collected in TINRO-Centre research surveys with a purpose to estimate the role of these cephalopods in pelagic communities Methods: – analyze database on squid catches for a period from 1990 to 2008; – use the data to obtain patterns of spatial and seasonal on squid distribution and biology; – assess the role of different species in seasonal dynamics of distribution density in the epi- and mesopelagic layers in different years; – use synoptic observations to reveal relationships between squid distribution density and variability in atmospheric pressure in the study area Upper-mesopelagic Region expeditions investigations years (expeditions) northern Okhotsk Sea 20 12 1990-2008 southern 18 2 1990-2008 Okhotsk Sea off west 12 7 1998-2007 Kamchatka the entire Okhotsk Sea 25 13 1990-2008 - Investigations of epipelagic (0-200 m) - Investigations of epipelagic and upper- mesopelagic (200-500 m) northern Okhotsk Sea Kamchatka Biomass density (kg/km2) of the Gonatidae in the northern Okhotsk Sea kg/km2 0,6 2 - Berryteuthis magister 0,5 4000,4 - Gonatus madokai 0,3 2000,2 0,1 0 w w s s о w s s о w s s о w s s о w s s о о w s s о w s s s о 1991 ] [ 1993][1994 1995 ][ 1997 1998 1999 2000 1,2 - Boreoteuthis borealis -

An Isotope Approach
Determining Cetacean – Cephalopod Trophic Interactions – An Isotope Approach By Christine Heather Jackson Bachelor of Arts, James Cook University Graduate Diploma (Health Promotion), Curtin University of Technology Master of Science, University of Tasmania Submitted in fulfilment of the requirements for the degree Doctor of Philosophy University of Tasmania (October 2016) ii DECLARATION This thesis contains no material which has been accepted for a degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright Signed; _________________________________ Christine H. Jackson iii AUTHORITY OF ACCESS This thesis may be made available for loan and limited copying and communication in accordance with the Copyright Act 1968. Signed; _________________________________ Christine H. Jackson iv ABSTRACT Determining the biotic and abiotic influences on the distribution and abundance of marine mammals is essential for understanding the dynamics of the food chain. The predator-prey relationship can be deterministic in shaping both the community structure and function of marine ecosystems. This is especially pertinent to recovering toothed whale populations given their large size and high prey consumption rates. A greater knowledge of the trophic linkages between toothed whales and their prey will facilitate assessments of their combined impact on the ecosystem since marine food webs are a fusion of bottom-up and top-down energy and nutrient flow. -

Redacted for Privacy William G
AN ABSTRACT OF THE THESIS OF Elizabeth H. Sinclair for the degree of Master of Science in Oceanography presented on December 16, 1988. Title: Feeding Habits of Northern Fur Seals (Callorhinus ursinus) in the Eastern Bering Sea Abstract approved: Redacted for privacy William G. Pearcy This study was conducted to determine the composition and size of prey consumed by northern fur seals (Callorhinus ursinus) in the eastern Bering Sea. Eighty three northern fur seals were collected in the summer and fall of 1981, 1982, and 1985 forexamination of gastrointestinal contents. A total of 139 midwater and bottom trawls were collected to determine the availability of potential prey. Analysis of trawls confirmed that seals are size-selective midwater feeders during their breeding and haul-out season in the eastern Bering Sea. Juvenile walleye pollock and gonatid squid, 5-20cm in body length, were the primary prey, but seal prey varied among years and between nearshore and pelagic sample locations. Interannual variation in body sizes of walleye pollock consumed by seals was related to pollock year class strength. The identification of pollock and gonatid squid as primary fur seal prey in the eastern Bering Sea was consistent with previous reports. However, Pacific herring and capelin, previously considered important fur seal prey werE absent in this study. Feeding Habits of Northern Fur Seals (Callorhinus ursinus) in the Eastern Bering Sea by Elizabeth Hacker Sinclair A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Completed December 16, 1988 Commencement June 1989 APPROVED: Redacted for privacy Professor of Oceanography in char.4f major Redacted for privacy Dean of C./lege of Ocean. -
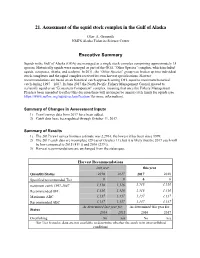
Assessment of the Squid Stock Complex in the Gulf of Alaska
21. Assessment of the squid stock complex in the Gulf of Alaska Olav A. Ormseth NMFS Alaska Fisheries Science Center Executive Summary Squids in the Gulf of Alaska (GOA) are managed as a single stock complex comprising approximately 15 species. Historically squids were managed as part of the GOA “Other Species” complex, which included squids, octopuses, sharks, and sculpins. In 2011, the “Other Species” group was broken up into individual stock complexes and the squid complex received its own harvest specifications. Harvest recommendations are based on an historical catch approach setting OFL equal to maximum historical catch during 1997 – 2007. In June 2017 the North Pacific Fishery Management Council moved to reclassify squid as an “Ecosystem Component” complex, meaning that once the Fishery Management Plan has been amended to reflect this decision there will no longer be annual catch limits for squids (see https://www.npfmc.org/squid-reclassification/ for more information). Summary of Changes in Assessment Inputs 1) Trawl survey data from 2017 have been added. 2) Catch data have been updated through October 11, 2017. Summary of Results 1) The 2017 trawl survey biomass estimate was 2,296 t, the lowest it has been since 1999. 2) The 2017 catch data are incomplete (29 t as of October 11), but it is likely that the 2017 catch will be low compared to 2015 (411 t) and 2016 (239 t). 3) Harvest recommendations are unchanged from the status quo. Harvest Recommendations last year this year Quantity/Status 2016 2017 2017 2018 Specified/recommended -

THE PINNIPEDS of the CALIFORNIA CURRENT California
ANTONELIS AND FISCUS: PINNIPEDS OF THE CALIFORNIA CURRENT CalCOFI Rep., Vol. XXI, 1980 THE PINNIPEDS OF THE CALIFORNIA CURRENT GEORGE A. ANTONELIS. JR. AND CLIFFORD H. FISCUS Marine Mammal Division Northwest and Alaska Fisheries Center National Marine Fisheries Service National Oceanic and Atmospheric Administration 7600 Sand Point Way, N.E. Seattle, WA 981 15 ABSTRACT 10s pequenos peces en 10s cardumenes y peces ana- There are six species of pinnipeds-California sea dromos. Los dos focidos, otra vez con ciertas excep- lion, Zalophus californianus; northern sea lion, Eume- ciones, predan especies diferentes. Aparentemente, el topias jubatus; northern fur seal, Callorhinus ursinus; elefante marino se alimenta en aguas mas profundas que Guadalupe fur seal, Arctocephalus townsendi; harbor la foca peluda, alimentindose de especies demersales seal, Phoca uitulina richardsi; and northern elephant y benticas, y la foca peluda se alimenta de especiesdemer- seal, Mirounga angustirostris-that inhabit the study sales costeras y neriticas, entrando ocasionalmente en rios area of the California Cooperative Oceanic Fisheries y aguas estuarinas haciendopresa de 10s peces anadromos Investigations (CalCOFI). y otros pequeiios peces que entran regularmente en estas The numbers of animals in each population are given; aguas. the size, distribution, and seasonal movements are de- scribed. The known prey species of the pinnipeds are INTRODUCTION listed for each species. The otariids, with certain excep- The California Current, its components, and the Cali- tions, consume the same kinds of prey, although in slight- fornia Cooperative Oceanic Fisheries Investigations ly different amounts. In general they feed most commonly (CalCOFI) station plan have been described many times on the smaller schooling fishes and squids of the epi- in the past and are well known (Kramer et al. -

A Review of Direct and Indirect Impacts of Marine Dredging Activities on Marine Mammals
A review of direct and indirect impacts of marine dredging activities on marine mammals Family Scientific name Common name Range of best Frequency of Minimum Methodology Diet Region Habitat Documented Effects of Potential Effects of Dredging (excluding (including hearing (10 dB minimum hearing Dredging subspecies) subspecies) from max; kHz) hearing threshold (dB threshold (kHz) re 1 µPa) Otariidae Arctocephalus Cape & Unknown; — — — Fish (e.g. Emmelichthys nitidus, F, J (Kirkman et Continental shelf waters (IUCN, — Habitat destruction, increase in pusillus Australian fur fundamental Pseudophycis bachus, Trachurus al., 2007; IUCN, 2012) turbidity, changes to prey seal frequency of declivis, Neoplatycephalus 2013; Perrin, availability, masking, incidental male in air barks Richardsoni) (Australian fur seal) 2013) capture or injury, avoidance & is 0.14 & female (Page et al., 2005) an increase in shipping traffic in air barks is 0.15 (Tripovich et al., 2008) Arctophoca Antarctic fur seal Unknown; peak — — — Fish (e.g. Gymnoscopelus A, F, J (IUCN, Forage in deep waters (>500 m) — Habitat destruction, increase in gazella frequency of in piabilis, Electrona subaspera, 2013; Perrin, with a strong chlorophyll turbidity, changes to prey air barks is 0.3– Champsocephalus gunnari) 2013; Reeves et concentration & steep availability, masking, incidental 5.9 (Page et al., (Guinet et al., 2001) al., 2002) bathymetric gradients, otherwise capture or injury, avoidance & 2002) remains close to the colony in an increase in shipping traffic areas with Polar -

Squids of the Family Onychoteuthidae Gray, 1847 in the Southeastern Pacific Ocean
Lat. Am. J. Aquat. Res., 44(2): 416-421, 2016 Onychoteuthid squids from Chile 416 1 DOI: 10.3856/vol44-issue2-fulltext-23 Short Communication Squids of the family Onychoteuthidae Gray, 1847 in the southeastern Pacific Ocean 1 1 Christian M. Ibáñez & Alina F. Cifuentes-Bustamante 1Departamento de Ecología y Biodiversidad, Facultad de Ecología y Recursos Naturales Universidad Andres Bello, Santiago, Chile Corresponding author: Christian M. Ibáñez ([email protected]) ABSTRACT. Hooked squids (Family Onychoteuthidae Gray, 1847) inhabit all oceans of the world except the Arctic. This family is currently comprised of 25 species belonging to seven genera. In the southeastern Pacific Ocean, approximately five onychoteuthid species have been previously identified, but true identity of these taxa is uncertain. We reviewed museum collections, from Chile, United States and New Zealand, and literature to elucidate the presence of hooked squids in the southeastern Pacific Ocean. The present status of the Onychoteuthidae from the southeastern Pacific only includes four species: Onychoteuthis aequimanus, Onykia ingens, Onykia robsoni, and Kondakovia nigmatullini. Keywords: Onychoteuthidae, Onychoteuthis, Onykia, Kondakovia, hooked squids, Chile. Calamares de la familia Onychoteuthidae Gray, 1847 en el Océano Pacífico suroriental RESUMEN. Los calamares ganchudos (Familia Onychoteuthidae Gray, 1847) habitan en todos los océanos excepto en el Ártico. Esta familia está compuesta actualmente de 25 especies pertenecientes a siete géneros. En el Océano Pacífico suroriental, aproximadamente cinco especies de Onychoteuthidae han sido identificadas previamente, pero su estatus taxonómico es incierto. Se revisaron las colecciones de museos de Chile, Estados Unidos y Nueva Zelanda, y la literatura para dilucidar la presencia de calamares con ganchos en el Pacífico suroriental. -

Cephalopod Beak Sections Used to Trace
Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study José Queirós, Paco Bustamante, Yves Cherel, Joao Coelho, José Seco, Jim Roberts, Eduarda Pereira, José Xavier To cite this version: José Queirós, Paco Bustamante, Yves Cherel, Joao Coelho, José Seco, et al.. Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study. Marine Environmental Research, Elsevier, 2020, 161, pp.105049. 10.1016/j.marenvres.2020.105049. hal-02904190 HAL Id: hal-02904190 https://hal.archives-ouvertes.fr/hal-02904190 Submitted on 21 Jul 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study José P. Queirós*a, Paco Bustamanteb,c, Yves Chereld, João P. Coelhoe, José Secof,g, Jim Robertsh, Eduarda Pereiraf & José C. Xaviera,i a – University of Coimbra, MARE – Marine and Environmental Sciences Centre, Department of Life Sciences, 3000-456, Coimbra, Portugal b - Littoral Environnement et Sociétés (LIENSs), UMR 7266 CNRS-La Rochelle Université, 2 rue Olympe de Gouges, 17000 La Rochelle, France c - Institut Universitaire de France (IUF), 1 rue Descartes 75005 Paris, France d - Centre d’Etudes Biologiques de Chizé, UMR 7372 du CNRS–La Rochelle Université, 79360 Villiers–en–Bois, France.