SPC Biological Sampling Newsletter-Issue#17-Jan11
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Technical Report No. 447 1974 •
FISHERIES RESEARCH BOARD OF CANADA TECHNICAL REPORT NO. 447 1974 • ... FISHERIES RESEARCH BOARD OF CANADA Technical Reports FRB Technical Reports are research documents that are of sufficient importance to be preserved, but which ·for some reason are not appropriate for primary scientific publication. No restriction is placed on subject matter and the series should reflect the broad research interests of FRB. These Reports can be cited in publications, but care should be taken to indicate their manuscript status. Some of the material in these Reports will eventually appear in the primary scientific literature. Inquiries concerning any particular Report should be directed to the issuing FRB establishment which is indicated on the title page. FISHERIES AND MARINE SERVICE TECHNICAL REPORT NO. 44 7 THE SQUID OF BRITISH COLUMBIA AS A POTENTIAL FISHERY RESOURCE - A PRELIMINARY REPORT by S.A. Macfarlane and M. Yamamoto Fisheries and Marine Service Vancouver Laboratory Vancouver, B.C. TABLE OF CONTENTS Page No. I. INTRODUCTION 1 II. BIOLOGICAL ASPECTS 3 III. COMMERCIAL ASPECTS 8 A. Fishing Methods 8 B. International Squid Fisher,y 15 c. Status of Squid in British Co1uabia 19 IV. NUTRITIONAL ASPECTS 27 v. PROCESSING 28 VI. DISCUSSION 30 VII. ACKNOWLEDGMENTS 32 VIII. REFERENCES 33 1. I. INTRODUCTION Available catch statistics from 1965 through 1971 indicate • that world-wide landings of squid totalled roughly 700,000 metric tons annually. An additional 100,000 metric tons of cuttlefish and about 160,000 metric tons of octopus were also landed annually. Apart from the well-established squid fishery in the Monterey area of California and the relatively minor inshore squid fishery off Newfoundland, the North American fishing industry has tended to ignore the possibility of further exploitation and utilization of this resource. -

<I>Onychoteuthis</I> Lichtenstein, 1818
BULLETIN OF MARINE SCIENCE, 83(3): 481–529, 2008 NEW TAXA PAPER TWO NEW SPECIES AND A REVIEW OF THE SQUID GENUS ONYCHOTEUTHIS LICHTENSTEIN, 1818 (OEGOPSIDA: ONYCHOTEUTHIDAE) FROM THE PACIFIC OCEAN K. S. Bolstad ABSTRACT The onychoteuthid genusOnychoteuthis Lichtenstein, 1818 is in systematic disarray. The oldest species, Onychoteuthis banksii (Leach, 1817), is widely recognized as a species complex, with which two of the other commonly recognized three species—Onychoteuthis compacta (Berry, 1913) and Onychoteuthis borealijaponica Okada, 1927—and some 20 additional names have been all synonymized at one time. This study, a partial revision of the genus, redescribes O. banksii from the Atlantic Ocean; from the Pacific, O. compacta, O. borealijaponica, and Onychoteuthis meridiopacificaRancurel and Okutani, 1990, are redescribed, the name Onychoteuthis aequimanus Gabb, 1868, is resurrected, and two additional species are described: Onychoteuthis lacrima sp. nov., and Onychoteuthis prolata sp. nov. Several new species-level characters are examined in detail, compared, and reported for each species, including buccal morphology, tentacular club and hook morphology, chromatophore patterns on the mantle and tentacles, and photophore shape and size. The cosmopolitan tropical/temperate genus Onychoteuthis has long been recog- nized for its systematic instability (Kubodera et al., 1998; Vecchione et al., 2003). Onychoteuthis banksii (Leach, 1817) has been a catch-all name for many morpho- logically similar taxa since its description nearly 200 yrs ago (Vecchione et al., 2003), including: two of the additional three species generally recognized by recent authors—Onychoteuthis compacta (Berry, 1913) and Onychoteuthis borealijaponica Okada, 1927, at various times—and about 20 additional nominal species (see e.g., Pfeffer, 1912; Adam, 1952). -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Vertical Distribution of Pelagic Cephalopods *
* Vertical Distribution of Pelagic Cephalopods CLYDE F. E. ROPER and RICHARD E. YOUNG SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 209 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Insti- tution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, com- mencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. These pub- lications are distributed by mailing lists to libraries, laboratories, and other interested institutions and specialists throughout the world. Individual copies may be obtained from the Smithsonian Institution Press as long as stocks are available. S. DILLON RIPLEY Secretary Smithsonian Institution SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 209 Vertical Distribution of Pelagic Cephalopds Clyde F. -

Squids of the Family Onychoteuthidae Gray, 1847 in the Southeastern Pacific Ocean
Lat. Am. J. Aquat. Res., 44(2): 416-421, 2016 Onychoteuthid squids from Chile 416 1 DOI: 10.3856/vol44-issue2-fulltext-23 Short Communication Squids of the family Onychoteuthidae Gray, 1847 in the southeastern Pacific Ocean 1 1 Christian M. Ibáñez & Alina F. Cifuentes-Bustamante 1Departamento de Ecología y Biodiversidad, Facultad de Ecología y Recursos Naturales Universidad Andres Bello, Santiago, Chile Corresponding author: Christian M. Ibáñez ([email protected]) ABSTRACT. Hooked squids (Family Onychoteuthidae Gray, 1847) inhabit all oceans of the world except the Arctic. This family is currently comprised of 25 species belonging to seven genera. In the southeastern Pacific Ocean, approximately five onychoteuthid species have been previously identified, but true identity of these taxa is uncertain. We reviewed museum collections, from Chile, United States and New Zealand, and literature to elucidate the presence of hooked squids in the southeastern Pacific Ocean. The present status of the Onychoteuthidae from the southeastern Pacific only includes four species: Onychoteuthis aequimanus, Onykia ingens, Onykia robsoni, and Kondakovia nigmatullini. Keywords: Onychoteuthidae, Onychoteuthis, Onykia, Kondakovia, hooked squids, Chile. Calamares de la familia Onychoteuthidae Gray, 1847 en el Océano Pacífico suroriental RESUMEN. Los calamares ganchudos (Familia Onychoteuthidae Gray, 1847) habitan en todos los océanos excepto en el Ártico. Esta familia está compuesta actualmente de 25 especies pertenecientes a siete géneros. En el Océano Pacífico suroriental, aproximadamente cinco especies de Onychoteuthidae han sido identificadas previamente, pero su estatus taxonómico es incierto. Se revisaron las colecciones de museos de Chile, Estados Unidos y Nueva Zelanda, y la literatura para dilucidar la presencia de calamares con ganchos en el Pacífico suroriental. -

Cephalopod Beak Sections Used to Trace
Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study José Queirós, Paco Bustamante, Yves Cherel, Joao Coelho, José Seco, Jim Roberts, Eduarda Pereira, José Xavier To cite this version: José Queirós, Paco Bustamante, Yves Cherel, Joao Coelho, José Seco, et al.. Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study. Marine Environmental Research, Elsevier, 2020, 161, pp.105049. 10.1016/j.marenvres.2020.105049. hal-02904190 HAL Id: hal-02904190 https://hal.archives-ouvertes.fr/hal-02904190 Submitted on 21 Jul 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Cephalopod beak sections used to trace mercury levels throughout the life of cephalopods: the Giant Warty squid Moroteuthopsis longimana as a case study José P. Queirós*a, Paco Bustamanteb,c, Yves Chereld, João P. Coelhoe, José Secof,g, Jim Robertsh, Eduarda Pereiraf & José C. Xaviera,i a – University of Coimbra, MARE – Marine and Environmental Sciences Centre, Department of Life Sciences, 3000-456, Coimbra, Portugal b - Littoral Environnement et Sociétés (LIENSs), UMR 7266 CNRS-La Rochelle Université, 2 rue Olympe de Gouges, 17000 La Rochelle, France c - Institut Universitaire de France (IUF), 1 rue Descartes 75005 Paris, France d - Centre d’Etudes Biologiques de Chizé, UMR 7372 du CNRS–La Rochelle Université, 79360 Villiers–en–Bois, France. -

University of Groningen Sperm Storage and Mating in the Deep-Sea
University of Groningen Sperm storage and mating in the deep-sea squid Taningia danae Joubin, 1931 (Oegopsida Hoving, Hendrik Jan T.; Lipinski, Marek R.; Videler, John J.; Bolstad, Kat S. R. Published in: Marine Biology DOI: 10.1007/s00227-009-1326-7 IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2010 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Hoving, H. J. T., Lipinski, M. R., Videler, J. J., & Bolstad, K. S. R. (2010). Sperm storage and mating in the deep-sea squid Taningia danae Joubin, 1931 (Oegopsida: Octopoteuthidae). Marine Biology, 157(2), 393- 400. https://doi.org/10.1007/s00227-009-1326-7 Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Fao Species Identification Sheets for Fishery Purposes
3D FAO SPECIES IDENTIFICATION SHEETS FOR FISHERY PURPOSES WESTERN CENTRAL ATLANTIC (Fishing Area 31) edited by W. Fischer Marine Resources Service Fishery Resources and Environment Division FAO Fisheries Department This publication has been prepared and printed with the support of the UNDP/FAO International Project for the Development of Fisheries in the Western Central Atlantic VOLUME VI CONTENTS: Lobsters Shrimps and Prawns True Crabs Stomatopods Bivalves Gastropods Chitons * Cephalopoda + Sea Turtles FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 1978 Bibliographic Reference: Roper, C.F.E. In: Fi.ch«, w. CEO cms) Rome, FAO, pag.var. FAO species identification sheets for fishery purposes. Western Central Atlantic (fishing area 31>. Vols. 1-7 Identification sheets. Taxonomy. Geographic distribution. Fisheries. Vernacular names. Bony fishes. Sharks. Batoid fishes. Lobsters. Shrimps. True crabs. S tomato pods. Molluscs. Sea turtles. ASH. FAO Sheets Fishing Area 3 I TECHNICAL TERMS lamellae I hectocotylus (or modified •< portion) suckers buccal membrane funnel groove oegopsid eye normal sucker: funne I funnel-mantle fusion funnel locking cartilage mantle locking cartilage 1 dactylus ~T"Y fin (posteriorly concave) _ fin (posteriorly V t convex) loss t— fin length example of hectocotylized arm in male (Illex ittecebrosue) __cail arm I (dorsal) mantle length buccal lappet J a composite diagram illustrating beak jaws basic squid (teuthoid) features ventral view buccal suckers buccal connective (ventrally attached) buccal connective (dorsally attached arm IV (ventral) diagram of oral surface of brachial crown and buccal membrane oval with inward simple, -^-shaped J_-shaped subtriangular projecting knobs straight basic types of funnel locking cartilage (cartilaginous grooves that lock with corresponding ridges on inner mantle wall to keep base of funnel in position during water expulsion) - 2 - FAO Sheets CEFHALOPOOS Fishing Area 31 mantle Length lame 1Lae (internal) / funnel aperture suckers V" total length A. -
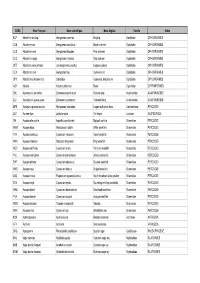
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa -

Redalyc.Squids of the Family Onychoteuthidae Gray, 1847 in The
Latin American Journal of Aquatic Research E-ISSN: 0718-560X [email protected] Pontificia Universidad Católica de Valparaíso Chile Ibáñez, Christian M.; Cifuentes-Bustamante, Alina F. Squids of the family Onychoteuthidae Gray, 1847 in the southeastern Pacific Ocean Latin American Journal of Aquatic Research, vol. 44, núm. 2, mayo, 2016, pp. 416-421 Pontificia Universidad Católica de Valparaíso Valparaíso, Chile Available in: http://www.redalyc.org/articulo.oa?id=175046298023 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Lat. Am. J. Aquat. Res., 44(2): 416-421, 2016 Onychoteuthid squids from Chile 416 1 DOI: 10.3856/vol44-issue2-fulltext-23 Short Communication Squids of the family Onychoteuthidae Gray, 1847 in the southeastern Pacific Ocean 1 1 Christian M. Ibáñez & Alina F. Cifuentes-Bustamante 1Departamento de Ecología y Biodiversidad, Facultad de Ecología y Recursos Naturales Universidad Andres Bello, Santiago, Chile Corresponding author: Christian M. Ibáñez ([email protected]) ABSTRACT. Hooked squids (Family Onychoteuthidae Gray, 1847) inhabit all oceans of the world except the Arctic. This family is currently comprised of 25 species belonging to seven genera. In the southeastern Pacific Ocean, approximately five onychoteuthid species have been previously identified, but true identity of these taxa is uncertain. We reviewed museum collections, from Chile, United States and New Zealand, and literature to elucidate the presence of hooked squids in the southeastern Pacific Ocean. The present status of the Onychoteuthidae from the southeastern Pacific only includes four species: Onychoteuthis aequimanus, Onykia ingens, Onykia robsoni, and Kondakovia nigmatullini. -

Kawamura, A. a Review of Food of Balaenopterid Whales. 155-197
A REVIEW OF FOOD OF BALAENOPTERID WHALES AKITO KAWAMURA Faculty of Fisheries, Hokkaido University, Hakodate, Hokkaido ABSTRACT In order to elucidate what species among so many kind of marine organ isms are likely to be consumed largely by the balaenopterid whales, the ex isting evidence on the food habits of baleen whales is reviewed. To meet with this primary purpose the report was mainly focussed on to describe qualitative aspects of food species having been known to date from the notable whaling grounds over the world rather than documenting quantitative subjects.' One of interesting facts noticed throughout the contribution was that there exists fairly intense diversity in the assembly of food species composition by regions such as; northern hemisphere vs. southern hemisphere, Pacific region vs. Atlantic region, inshore waters vs. offshore waters, embayed waters vs. open waters, where the former usually shows more div'ersed complexity than the latter. The fact however suggests that although the composition of food spe cies locally varies over the various whaling grounds, the food organisms as taxonomical groups are very similar one another even in locally isolated whal ing grounds when the food organisms and their assemblies are considered by the family or genus basis. In this connection many evidences given in the text may suggest that the balaenopterid whales as a whole may substantially live on quite simply compositioned forage assembly in comparison with tre mendous variety of organisms existing in the marine ecosystems. One of im portant aspects of the baleen whales food must be found in their characteris tics of forming dense swarms, schools, and/or aggregations in the shallower enough layers to be fed by the whales.