The Evolution of Hydrothermal Fluids from the Deep Porphyry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gamonal S.Pdf
Indice 1. Introducción …………………………………………………………………………. 6 1.1 Objetivos ………………………………………………………………...................... 7 1.2 Ubicación y accesos ..………………………………………………………………… 8 1.3 Clima y vegetación ………………………………………………………………….. 10 1.4 Metodología …………………………………………………………………………. 10 1.5 Historia de la propiedad y trabajos anteriores ……………………………………….. 11 2. Marco Geológico Regional ………………………………………………………...... 12 2.1 Basamento …………………………………………………………………………… 12 2.2 Volcanismo Cenozoico ……………………………………………………………… 14 2.3 Tectónica y estructuras ………………………………………………………………. 15 2.4 Alteración y mineralización ………………………………………………………… 18 3. Geología local ………………………………………………………………………… 19 3.1 Rocas estratificadas e intrusivas …………………………………………………….. 20 3.1.1 Formación Pantanoso (Pz) …………………………………………………...... 21 3.1.2 Lavas de Quebrada de Tapia (Kt) ……………………………………………… 21 3.1.3 Formación Astaburuaga (FAs) ………………………………………………… 21 3.1.4 Complejos de domos y depósitos volcánicos asociados (CDDV)………………. 23 3.1.4.1 Depósitos volcánicos y volcanoclásticos (CDv) …………………………… 23 3.1.4.2 Cuerpos Intrusivos (CDIn) ……………………………………………….. 24 3.1.4.3 Brechas freatomagmáticas (Bfm) ………………………………………… 25 3.1.5 Estratos de Sierra de la Sal (ESS) ……………………………………………… 26 3.1.6 Unidad Ignimbrítica I (UIg1) ………………………………………………….. 27 3.1.7 Unidad Tobácea (UTo) ………………………………………………………… 27 3.1.8 Unidad Andesítica Superior (UAS) …………………………………………… 28 3.1.9 Unidad Ignimbrítica II (UIg2) …………………………………………………. 29 3.2 Depósitos No consolidados ………………………………………………………… 29 3.2.1 Depósitos Aluviales de gravas -

Biofilm Adhesion on the Sulfide Mineral Bornite & Implications for Astrobiology
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2019 BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY Margaret M. Wilson University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Wilson, Margaret M., "BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY" (2019). Open Access Master's Theses. Paper 1517. https://digitalcommons.uri.edu/theses/1517 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY BY MARGARET M. WILSON A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE UNIVERSITY OF RHODE ISLAND 2019 MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE THESIS OF MARGARET M. WILSON APPROVED: Thesis Committee: Major Professor Dawn Cardace José Amador Roxanne Beinart Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2019 ABSTRACT We present research observing and documenting the model organism, Pseudomonas fluorescens (P. fluorescens), building biofilm on a natural mineral substrate composed largely of bornite (Cu5FeS4), a copper-iron sulfide mineral, with closely intergrown regions of covellite (CuS) and chalcopyrite (CuFeS2). In examining biofilm establishment on sulfide minerals, we investigate a potential habitable niche for microorganisms in extraterrestrial sites. Geochemical microenvironments on Earth and in the lab can also serve as analogs for important extraterrestrial sites, such as sheltered, subsurface microenvironments on Mars. -

Muntean/Einaudi
Economic Geology Vol. 95, 2000, pp. 1445–1472 Porphyry Gold Deposits of the Refugio District, Maricunga Belt, Northern Chile JOHN L. MUNTEAN†,* AND MARCO T. EINAUDI Department of Geological and Environmental Sciences, Stanford University, Stanford, California 94305-2115 Abstract The porphyry gold deposits of the Refugio district and similar deposits in the Maricunga belt contain the lowest known copper to gold ratios (% Cu/ppm Au = ~0.03) of any porphyry-type deposit. The gold deposits are associated with subvolcanic andesitic to dacitic intrusions emplaced into coeval volcanic rocks. Both the Verde and Pancho deposits are zoned in space from a deeper zone of banded quartz veinlets associated with chlorite-magnetite-albite and/or pyrite-albite-clay alteration to a shallow zone of pyrite-albite-clay and local quartz-alunite ledges. Pancho contains an additional, deepest, porphyry copperlike zone, with quartz veinlets (A-veinlets) and potassic alteration. Relative to Verde, Pancho is telescoped, with all three zones present within a 400-m-vertical interval. The porphyry copperlike zone at Pancho is characterized by A-veinlets and pervasive potassic alteration, both restricted to intrusive rocks. A-veinlets range from hairline streaks of magnetite ± biotite with minor quartz and chalcopyrite, and K feldspar alteration envelopes to sugary quartz veinlets <1 cm in width with mag- netite and chalcopyrite and no alteration envelopes. Hypersaline liquid inclusions coexisting with vapor-rich in- clusions indicate temperatures above 600°C and salinities as high as 84 wt percent NaCl equiv. A pressure es- timate of 250 bars indicates a depth of 1,000 m, assuming lithostatic pressure. -

Selective Flotation of Enargite from Copper Sulphides in Complex Ore Systems
Selective flotation of enargite from copper sulphides in complex ore systems Maedeh Tayebi-Khorami M.Sc. (Mineral Processing Engineering) B.Sc. (Mining Engineering) A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in November 2016 Sustainable Minerals Institute Abstract Recent research has demonstrated promising results showing the possibility of separating arsenic- copper sulphides from other copper minerals by controlling the potential of the flotation pulp. Most of these studies were conducted on single mineral systems, and the selective removal of arsenic- copper minerals in real ore systems is not well understood. In real ore systems, the efficiency of the separation strongly depends on the mineralogical characteristics of the ore samples. This study seeks to understand the effect of ore mineralogy on the floatability of enargite in a complex ore system, under a controlled potential flotation environment. A composite of several high arsenic-containing drill core intersections for the high arsenic sample (HAS) and a composite of some low arsenic-containing drill core intersections for the low arsenic sample (LAS) were selected from the Tampakan copper-gold deposit in the Philippines, providing a range of arsenic levels. Arsenic in the HAS sample (enargite) was practically twice that for the LAS sample. The non-enargite copper minerals (NECu) were mostly chalcopyrite and bornite in both samples. Comprehensive size-by-size, chemical and mineralogical analyses were performed on both ore samples. It was observed that the two ore samples had similar mineralogical characteristics in terms of mineral content and liberation distribution, however there are some differences in the proportions of minerals. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Atacama Pacific Gold Corporation
TECHNICAL REPORT on the CERRO MARICUNGA GOLD PROJECT Region III CHILE prepared for ATACAMA PACIFIC GOLD CORPORATION 330 Bay Street, Suite 1210, Toronto, Ontario Canada M5H 2S8 October 7, 2011 Prepared By: Michael Easdon, Oregon Reg. Prof. Geologist Alcántara 1128, Depto. 905, Las Condes Santiago, Chile [email protected] TABLE OF CONTENTS 1.0 SUMMARY ........................................................................................................... 1 2.0 INTRODUCTION .................................................................................................. 5 3.0 RELIANCE ON OTHER EXPERTS ...................................................................... 7 4.0 PROPERTY DESCRIPTION AND LOCATION ..................................................... 8 5.0 ACCESSIBILITY, CLIMATE, LOCAL RESOURCES, INFRASTRUCTURE AND PHYSIOGRAPHY ............................................................................................... 13 6.0 HISTORY ........................................................................................................... 15 7.0 GEOLOGICAL SETTING AND MINERALIZATION ............................................ 15 7.1 PROPERTY GEOLOGY: ............................................................................. 16 8.0 DEPOSIT TYPE ................................................................................................. 33 9.0 EXPLORATION .................................................................................................. 34 10.0 DRILLING .......................................................................................................... -

Magma Emplacement and Deformation in Rhyolitic Dykes: Insight Into Magmatic Outgassing
MAGMA EMPLACEMENT AND DEFORMATION IN RHYOLITIC DYKES: INSIGHT INTO MAGMATIC OUTGASSING Presented for the degree of Ph.D. by Ellen Marie McGowan MGeol (The University of Leicester, 2011) Initial submission January 2016 Final submission September 2016 Lancaster Environment Centre, Lancaster University Declaration I, Ellen Marie McGowan, hereby declare that the content of this thesis is the result of my own work, and that no part of the work has been submitted in substantially the same form for the award of a higher degree elsewhere. This thesis is dedicated to Nan-Nar, who sadly passed away in 2015. Nan, you taught our family the importance and meaning of love, we love you. Abstract Exposed rhyolitic dykes at eroded volcanoes arguably provide in situ records of conduit processes during rhyolitic eruptions, thus bridging the gap between surface and sub-surface processes. This study involved micro- to macro-scale analysis of the textures and water content within shallow (emplacement depths <500 m) rhyolitic dykes at two Icelandic central volcanoes. It is demonstrated that dyke propagation commenced with the intrusion of gas- charged currents that were laden with particles, and that the distribution of intruded particles and degree of magmatic overpressure required for dyke propagation were governed by the country rock permeability and strength, with pre-existing fractures playing a pivotal governing role. During this stage of dyke evolution significant amounts of exsolved gas may have escaped. Furthermore, during later magma emplacement within the dyke interiors, particles that were intruded and deposited during the initial phase were sometimes preserved at the dyke margins, forming dyke- marginal external tuffisite veins, which would have been capable of facilitating persistent outgassing during dyke growth. -

Nombre Entidad DOMICILIO COMUNA REGIÓN TRAMITACION Registro Public
N° Fecha Fecha # ESTADO DE LA Regi N° Diario Nombre Entidad DOMICILIO COMUNA REGIÓN TRAMITACION Registro Public. stro MISION EVANGÉLICA PENTECOSTAL EL PEDRO URRIOLA N° 1290 - REGIÓN 1 TERMINADA 2 14-12-99 22-02-01 36895 CERRO NAVIA PESEBRE HUMILDE DE CRISTO CERRO NAVIA METROPOLITANA REGIÓN DEL BIO- 2 TERMINADA 4 15-06-00 13-10-00 36786 "CORPORACIÓN EVANGÉLICA ECO MUNDIAL" POBLA. LA ALBORADA, CALLE 2 Nº 29 CONCEPCION BIO SAN PABLO N.8223, BLOCK REGIÓN 3 TERMINADA 6 17-07-00 21-04-01 36943 APOSTOLES DE LOS ULTIMOS TIEMPOS SAN RAMÓN 44, DEPTO 201. LO PRADO METROPOLITANA AVDA. PEDRO DE VALDIVIA REGIÓN 4 TERMINADA 8 10-08-00 11-11-00 36810 IGLESIA EVANGÉLICA LUTERANA EN CHILE SANTIAGO Nº 3420, OF. 33 METROPOLITANA CALLE SCHUYLER Nº 77, REGIÓN DEL BIO- 5 TERMINADA 10 17-08-00 21-03-01 36918 IGLESIA EVANGÉLICA EPISCOPAL MISIONERA TALCAHUANO POBLA. PARTAL BIO CORPORACIÓN EVANGÉLICA "PLENITUD DE FRANCISCO DE ARANDA Nº REGIÓN 6 TERMINADA 13 22-08-00 14-05-01 36961 SAN BERNARDO CRISTO" 659 - A - SAN BERNARDO METROPOLITANA FERNANDO LAZCANO Nº REGIÓN 7 TERMINADA 14 22-08-00 22-01-01 36868 "IGLESIA EVANGÉLICA PENTECOSTAL" SAN MIGUEL 1298 - SAN MIGUEL METROPOLITANA CALLE GUAYANAS Nº 350, REGIÓN DE 8 TERMINADA 15 22-08-00 17-04-01 36939 IGLESIA EVANGÉLICA PENTECOSTAL DE CRISTO LOS ANDES POBLA. CENTENARIO VALPARAISO CALLE J. ZAMORE, JM. REGIÓN DE 9 TERMINADA 16 23-08-00 21-08-02 37339 IGLESIA EVANGELISTICA EL SEMBRADOR CARO Y PASAJE UNO N° 51, VALPARAISO VALPARAISO 1° SECTOR DE PLAYA ANCHA PASAJE SPICA 249 - REGIÓN 10 TERMINADA 21 11-09-00 20-01-10 39864 "UNIDOS EN CRISTO" PUDAHUEL PUDAHUEL METROPOLITANA POBL. -
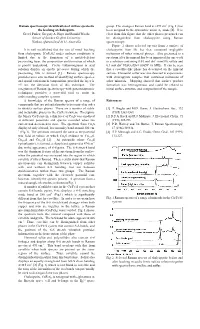
Raman Spectroscopic Identification of Surface Species in the Leaching Of
Raman spectroscopic identification of surface species in group. The strongest Raman band at »293 cm-1 (Fig. 1) has the leaching of chalcopyrite been assigned to the symmetric anion A1 mode [4]. It is Gretel Parker, Gregory A. Hope and Ronald Woods clear from this figure that the other phases presented can School of Science Griffith University be distinguished from chalcopyrite using Raman Nathan, Queensland 4111, Australia spectroscopy. Figure 2 shows selected spectra from a surface of It is well established that the rate of metal leaching chalcopyrite from Mt Isa that contained negligible from chalcopyrite [CuFeS2] under ambient conditions is inclusions of other mineral phases. Also presented is a limited due to the formation of a metal-deficient spectrum after the mineral has been immersed for one week passivating layer, the composition and formation of which in a solution containing 0.03 mol dm-3 iron(III) sulfate and -3 is poorly understood. Cyclic voltammograms in acid 0.1 mol dm H2SO4 (Eh = 0.865V vs SHE). It can be seen solution display an anodic pre-wave during which the that a covellite-like phase has developed on the mineral passivating film is formed [1]. Raman spectroscopy surface. Elemental sulfur was also detected in experiments provides an in situ method of identifying surface species, with chalcopyrite samples that contained inclusions of and spatial variations in composition, provided the layer is other minerals. Mapping showed that surface product >5 nm, the detection limit of this technique. The formation was heterogeneous and could be related to integration of Raman spectroscopy with potentiodynamic initial surface structure and composition of the sample. -

Supergene Mineralisation of the Boyongan Porphyry Copper-Gold Deposit, Surigao Del Norte, Philippines
Supergene Mineralisation of the Boyongan Porphyry Copper-Gold Deposit, Surigao del Norte, Philippines by Allan Maglaya Ignacio B.Sc. Geology, National Institute of Geological Sciences University of the Philippines Thesis submitted in partial fulfilment of the requirements of the Masters of Economic Geology Degree Centre for Ore Deposit Research, University of Tasmania December, 2005 DECLARATION OF ORIGINALITY This thesis contains no material which has been accepted for a degree of diploma by the University of Tasmania or any other institution, except by way of background information and duly acknowledged in the thesis, and contains no previous material previously pub- lished or written by another person except where due acknowledgement is given. Allan Maglaya Ignacio 01 December 2005 _________________________ STATEMENT OF AUTHORITY OF ACCESS This thesis may not to be made available for loan or copying for 1.5 years following the date this statement was signed. Following that time, the thesis may be available for loan and lim- ited copying in accordance with Copyright Act 1968. Allan Maglaya Ignacio 01 December 2005 _________________________ TABLE OF CONTENTS Page (s) LIST OF FIGURES …………………………………………………….. i - iii LIST OF APPENDICES ………………………………………………… iv ACKNOWLEDGMENTS ………………………………………………. v ABSTRACT ……………………………………………………………... vi - vii 1.0 INTRODUCTION ………………………………………………………. 1 - 8 1.1 Introduction …………………………………………………………. 1 1.2 Aims and Objectives ……………………………………………….. 1 1.3 Methods Employed …………………………………………………. 2 1.4 Location and Accessibility …………………………………………. 3 1.5 Climate ……………………………………………………………... 5 1.6 Previous Work ……………………………………………………… 5 2.0 GEOLOGICAL SETTING ………………………………………………. 9 - 37 2.1 Introduction ………………………………………………………. 9 2.2 Regional Tectonics …………….…………………………………. 9 2.3 Regional and Local Stratigraphy ………………………………... 11 2.3.1 Basement (Cretaceous-Paleogene) ………………………. 11 2.3.2 Bacuag Formation (Oliogocene-Miocene) .…………….. -

AN OCCURRENCE of the ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.S"", AUCANQUILCHA, CHILE Aran H. Cr-Etr&L
THE AMERICAN MINERALOGIST, VOL. 55, MAY_JUNE, 1970 AN OCCURRENCE OF THE ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.s"",AUCANQUILCHA, CHILE AraN H. Cr-etr<, Deportmentof GeologicalSciences, Queen's U niaersity, Kingston, Ontario. AssrnA.cr A mineral with composition near to CusFeSo has been found associated with nzrtive sulfur and covellite in the volcanic sulfur deposit at Aucanquilcha. Microprobe, optical and X-ray powder diffraction data match closell'a previously reported occurrence at Nu- kundamu, Fiji. Comparison with equilibrium synthesis by Kullerud and others indir:ates formation in the temperature range 434-482"C. INrnonucrroN Electron probe microanalysis(L6vy, 1967; Sillitoe and Clark, lt69) of naturally-occurring,supergene idaite has cast considerabledoub1. on the equation (Frenzel, 1959) of this not uncommon sulfi.de with the phaseof generalformula Cur r,Fe,Se.s,1 (Yund, 1963), which has h,een synthesizedby Merwin and Lombard (1937), Roseboomand Kullerud (1958),and Yund and Kullerud (1966).Idaite has been shown to have the compositionCurFeSa, or Cu3FeS4-,1orrd there is as yet no evidence of solid solution in nature between this and more copper-rich comF,osi- tions.The well-establishedX-ray powder data"(a:3.772 A; c:11.1U A; Yund, 1963) for hexagonal Cus.s,Fe"Se.r, are only with difficulty recon- ciled with thoseof natural idaite (Frenzel,1959, 1963), and L6vy (I\167) has suggestedthat Frenzel'soriginal powder data may be adequately fitted to a stannite-type,tetragonal cell. A differencein crystal struclure between thesephases is supported by the dissimilar reflectivity disper- sion profi.lesof natural idaite (L6vy, 1967; Sillitoe and Clark, 1969) and the synthetic "Cu5FeS6"of Merwin and l-ombard (1937; inLlvy, 1967). -

Multi-Stage Arc Magma Evolution Recorded by Apatite in Volcanic Rocks Chetan L
https://doi.org/10.1130/G46998.1 Manuscript received 6 February 2019 Revised manuscript received 11 November 2019 Manuscript accepted 5 December 2019 © 2020 The Authors. Gold Open Access: This paper is published under the terms of the CC-BY license. Published online 17 January 2020 Multi-stage arc magma evolution recorded by apatite in volcanic rocks Chetan L. Nathwani1,2, Matthew A. Loader2, Jamie J. Wilkinson2,1, Yannick Buret2, Robert H. Sievwright2 and Pete Hollings3 1 Department of Earth Science and Engineering, Imperial College London, Exhibition Road, South Kensington Campus, London SW7 2AZ, UK 2 London Centre for Ore Deposits and Exploration (LODE), Department of Earth Sciences, Natural History Museum, Cromwell Road, South Kensington, London SW7 5BD, UK 3 Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT However, accessory minerals such as apatite are Protracted magma storage in the deep crust is a key stage in the formation of evolved, capable of capturing discrete periods of melt hydrous arc magmas that can result in explosive volcanism and the formation of economi- evolution during differentiation. For example, cally valuable magmatic-hydrothermal ore deposits. High magmatic water content in the apatite has been shown to record the Sr con- deep crust results in extensive amphibole ± garnet fractionation and the suppression of pla- tent of the melt at the time of its crystallization, gioclase crystallization as recorded by elevated Sr/Y ratios and high Eu (high Eu/Eu*) in the which has been used to reconstruct host-rock melt. Here, we use a novel approach to track the petrogenesis of arc magmas using apatite compositions in provenance studies (Jennings trace element chemistry in volcanic formations from the Cenozoic arc of central Chile.