Controlled Chemicals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

The Detection and Determination of Esters
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1958 The etD ection and Determination of Esters. Mohd. Mohsin Qureshi Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Qureshi, Mohd. Mohsin, "The eD tection and Determination of Esters." (1958). LSU Historical Dissertations and Theses. 501. https://digitalcommons.lsu.edu/gradschool_disstheses/501 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. Copright by Mohcl Mohsin Qureshi 1959 THE DETECTION AND DETERMINATION OF ESTERS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Mohd. Mohsin Qureshi M.Sc., Aligarh University, 1944 August, 1958 ACKNOWLEDGMENT The author wishes to express his sincere apprecia tion and gratitude to Dr. Philip W. West under whose guidance this research was carried out. He is grateful to Dr. James G. Traynham for sup plying him with a number of esters and for his many helpful suggestions. The financial support given to him by the Continental Oil Company is gratefully acknowledged. He offers his sincere thanks to Miss Magdalena Usategul for her valuable suggestions and her ungrudging help during the course of this investigation. Dr. Anil K. -

Scientific Advisory Board
OPCW Scientific Advisory Board SAB-III/1 27 April 2000 Original: ENGLISH REPORT OF THE THIRD SESSION OF THE SCIENTIFIC ADVISORY BOARD 1. Introduction 1.1 The Scientific Advisory Board (hereinafter referred to as the “Board”) held two meetings during its third session, which took place from 14 - 16 December 1999 and from 15 - 16 March 2000 in The Hague. 1.2 Dr Claude Eon of France, the Chairman of the Board, presided over its proceedings. 1.3 The Board considered the following issues: (a) adamsite; (b) analytical procedures; (c) equipment issues; (d) destruction technologies; (e) bio-medical samples; (f) future contributions of the Board to the preparation of the first Review Conference; and (g) any other business. 1.4 In preparation for its meeting the Board had received reports from its temporary working groups (TWGs) on adamsite and analytical procedures, and a report on a joint meeting of the temporary working groups on equipment issues and on chemical weapons destruction technologies. 1.5 During its meeting in December 1999, the Board received a briefing by the Deputy Director-General on the status of implementation of the Convention and on work priorities. The Board was also briefed by staff from the Verification and Inspectorate Divisions on experiences with the conduct of different types of inspection, as well as on analytical and equipment-related matters. It was further briefed on the results of an expert meeting on bio-medical samples conducted by the Secretariat in December 1999. CS-2000-1867 SAB-III/1 page 2 2. Work on substantive issues Adamsite 2.1 The Board received and discussed the draft report of the TWG on adamsite dated 7 October 1999, noted additional comments, and decided to include in its report the following considerations in relation to this matter: 2.2 Adamsite (10-chloro-5,10-dihydro-phenarsazine, code name DM, CAS registry number 578-94-9) is a yellow-green crystalline solid with a boiling point of 410ºC and a melting point of 195ºC. -

Copyrighted Material
1 Historical Milieu 1.1 Organophosphorus Nerve Agents 2 1.2 Blister Agents 5 1.3 Sternutator Agents 11 1.4 Chemical Weapons Convention (CWC) 13 1.4.1 Schedule of Chemicals 14 1.4.2 Destruction of Chemical Weapons 14 References 16 COPYRIGHTED MATERIAL Analysis of Chemical Warfare Degradation Products, First Edition. Karolin K. Kroening, Renee N. Easter, Douglas D. Richardson, Stuart A. Willison and Joseph A. Caruso. © 2011 John Wiley & Sons, Ltd. Published 2011 by John Wiley & Sons, Ltd. 2 ANALYSIS OF CHEMICAL WARFARE DEGRADATION PRODUCTS 1.1 ORGANOPHOSPHORUS NERVE AGENTS Organophosphorus (OP) type compounds, that is, deriva- tives containing the P=O moiety, were first discovered in the 1800s when researchers were investigating useful applica- tions for insecticides/rodenticides. There are many derivatives of organophosphorus compounds, however, the OP deriva- tives that are typically known as ‘nerve agents’ were discov- ered accidentally in Germany in 1936 by a research team led by Dr. Gerhard Schrader at IG Farben [1–4]. Schrader had noticed the effects and lethality of these organophosphorus compounds towards insects and began developing a new class of insecticides. While working towards the goal of an improved insecticide, Schrader experimented with numerous phosphorus-containing compounds, leading to the discovery of the first nerve agent, Tabun (or GA) (Figure 1.1). The potency of these insecticides towards humans was not realized until there was yet another accident, which involved a Tabun spill. Schrader and coworkers began experiencing symptoms, such as miosis (constriction of the pupils of the eyes), dizziness and severe shortness of breath, with numerous effects lasting several weeks [1, 4, 5]. -

Chemical Warfare Agent (CWA) Identification Overview
Physicians for Human Rights Chemical Warfare Agent (CWA) Identification Overview Chemical Warfare Agent Identification Fact Sheet Series Table of Contents This Chemical Warfare Agent (CWA) Identification Fact Sheet is part 2 Physical Properties of a Physicians for Human Rights (PHR) series designed to fill a gap in 2 VX (Nerve Agent) 2 Sarin (Nerve Agent) knowledge among medical first responders to possible CWA attacks. 2 Tabun (Nerve Agent) This document in particular outlines differences between a select 2 BZ (Incapacitating Agent) group of vesicants and nerve agents, the deployment of which would 2 Mustard Gas (Vesicant) necessitate emergency medical treatment and documentation. 3 Collecting Samples to Test for Exposure 4 Protection PHR hopes that, by referencing these fact sheets, medical professionals 5 Symptoms may be able to correctly diagnose, treat, and document evidence of 6 Differential Diagnosis exposure to CWAs. Information in this fact sheet has been compiled from 8 Decontimanation 9 Treatment publicly available sources. 9 Abbreviations A series of detailed CWA fact sheets outlining in detail those properties and treatment regimes unique to each CWA is available at physiciansforhumanrights.org/training/chemical-weapons. phr.org Chemical Warfare Agent (CWA) Identification Overview 1 Collect urine samples, and blood and hair samples if possible, immediately after exposure Physical Properties VX • A lethal dose (10 mg) of VX, absorbed through the skin, can kill within minutes (Nerve Agent) • Can remain in environment for weeks -

Chlorine.Pdf
Chlorine 7782-50-5 Hazard Summary Chlorine is a commonly used household cleaner and disinfectant. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. Chronic (long-term) exposure to chlorine gas in workers has resulted in respiratory effects, including eye and throat irritation and airflow obstruction. No information is available on the carcinogenic effects of chlorine in humans from inhalation exposure. A National Toxicology Program (NTP) study showed no evidence of carcinogenic activity in male rats or male and female mice, and equivocal evidence in female rats, from ingestion of chlorinated water. EPA has not classified chlorine for potential carcinogenicity. Please Note: The main sources of information for this fact sheet are EPA's Integrated Risk Information System (IRIS) (2), which contains information on oral chronic toxicity and the RfD, The California Environmental Protection Agency's (CalEPA's) Technical Support Document for the Determination of Noncancer Chronic Reference Exposure Levels (3), and EPA's Drinking Water Criteria Document for Chlorine, Hypochlorous Acid and Hypochlorite Ion (1). Uses Chlorine is a commonly used household cleaner and disinfectant. It is widely used as an oxidizing agent in water treatment and chemical processes. It is also used in the bleaching process of wood pulp in pulp mills. (8) Sources and Potential Exposure Workers may be exposed to chlorine in industries where it is produced or used, particularly in the food and paper industries. In addition, persons breathing air around these industries may be exposed to chlorine. (1) Exposure to chlorine may also occur through drinking water and swimming pool water, where it is used as a disinfectant. -
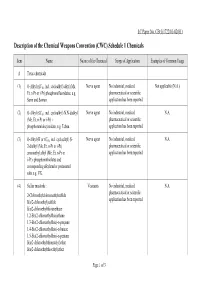
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

Report on Chemical Munitions Dumped in the Baltic Sea (HELCOM 1994)
Baltic Sea Environment Proceedings No. 142 Baltic Marine Environment Protection Commission Chemical Munitions Dumped in the Baltic Sea Published by: HELCOM – Baltic Marine Environment Protection Commission Katajanokanlaituri 6 B FI-00160 Helsinki Finland www.helcom.fi Authors: Tobias Knobloch (Dr.), Jacek Bełdowski, Claus Böttcher, Martin Söderström, Niels-Peter Rühl, Jens Sternheim For bibliographic purposes this document should be cited as: HELCOM, 2013 Chemical Munitions Dumped in the Baltic Sea. Report of the ad hoc Expert Group to Update and Review the Existing Information on Dumped Chemical Munitions in the Baltic Sea (HELCOM MUNI) Baltic Sea Environment Proceeding (BSEP) No. 142 Number of pages: 128 Information included in this publication or extracts thereof are free for citation on the condition that the complete reference of the publication is given as stated above Copyright 2013 by the Baltic Marine Environment Protection Commission (HELCOM) ISSN 0357-2994 Language revision: Howard McKee Editing: Minna Pyhälä and Mikhail Durkin Design and layout: Leena Närhi, Bitdesign, Vantaa, Finland Chemical Munitions Dumped in the Baltic Sea Report of the ad hoc Expert Group to Update and Review the Existing Information on Dumped Chemical Munitions in the Baltic Sea (HELCOM MUNI) Table of Contents 1 Executive summary. .5 2 Introduction. .9 2.1 CHEMU report – subjects covered, recommendations & fulfilment. .10 2.2 MUNI report – scope & perspectives. 11 2.3 National and international activities since 1995. .14 2.3.1 Managerial initiatives. .14 2.3.2 Investigations in the Baltic Sea . .23 3 Chemical warfare materials dumped in the Baltic Sea. .28 3.1 Introduction. 29 3.1.1 Dumping activities . -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention. -

Argonne Report.Pdf
CONTENTS NOTATION ........................................................................................................................... xi ABSTRACT ........................................................................................................................... 1 1 INTRODUCTION ........................................................................................................... 5 1.1 Overview of the Emergency Response Guidebook ................................................ 5 1.2 Organization of this Report ..................................................................................... 7 2 GENERAL METHODOLOGY ....................................................................................... 9 2.1 TIH List ................................................................................................................... 10 2.1.1 Background ................................................................................................. 10 2.1.2 Changes in the TIH List for the ERG2012 ................................................. 11 2.2 Shipment and Release Scenarios ............................................................................ 11 2.2.1 Shipment Profiles ........................................................................................ 12 2.2.2 Treatment of Chemical Agents ................................................................... 14 2.3 Generics, Mixtures, and Solutions .......................................................................... 17 2.4 Analysis of Water-Reactive -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Technical Note 159 Chemical Warfare Agent Measurements By
Technical Note TN-159 03/06/WH CHEMICAL WARFARE AGENT MEASUREMENTS BY PID INTRODUCTION nerve agents at these levels. However, it can locate sources and Many chemical warfare agents, including nerve agents and related detect the agents at levels well below levels that are lethal in one compounds, can be detected by PID. Table 1 lists some common minute (see LCy 50 in table 1). Compounds with low vapor pressures agents and several of their physical properties and PID Correction tend to respond more slowly on the PID, in some cases requiring Factors (CF). The CF is used by calibrating the instrument with several minutes. In the case of VX, the lethal dose is above its vapor isobutylene, and then multiplying the reading by the CF to obtain the pressure at room temperature. There fore, the lethal one-minute true concentration. (See Technical Note TN-106 for full details.) dose can be attained only if the air is hot or the chemical is sprayed as an aerosol. At the maximum concentration, more than one- DISCUSSION AND CONCLUSIONS minute exposure is required for lethal effects. All the warfare agents listed in Table 1 can be detected with a 10.6 Table 2 shows that many of the common decomposition products eV lamp, except phosgene, which requires an 11.7 eV lamp, and of aged warfare agents can also bedetected by PID. These are HCN and ClCN, which cannot be detected by PID. often more volatile than the agent itself (especially for VX) and thus VX has inherent sensitivity, but it is too heavy a compound to get the products serve as a more easily detectable surrogate than the to the PID sensor and thus cannot be reliably measured.