National Forest Genetic Electrophoresis Lab Annual Report 2003 – 2004 (FY04)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CIP Report 2001-02
Fort Ord Reuse Authority Capital Improvement Program (CIP) FY 2001/2002 through 2020/2021 (Final Version – FORA Board Approval, 06/08/01) 1 Table of Contents Page No. I. Executive Summary 3, 4, 5 II. Obligatory Program of Projects – Description of CIP Elements a. Transportation/Transit Projects 6, 7, 8, 9 b. Potable Water Augmentation 10 c. Storm Drainage System Projects 11 d. Habitat Management Requirements 12 e. Public Facility (Fire Station) Requirements 13 f. Building Removal Program 14, 15, 16 g. Water and Wastewater Collection Systems 17 III. FY 2001/2002 through 2020/2021 CIP a. Transportation/Transit Element 18-22 b. Summary of Obligatory CIP Elements 23, 24 c. Summary Spreadsheet (Overall CIP) 25 Appendices A. Protocol for Review/Reprogramming of CIP 26 B. Summary of funded projects through 2000/2001 27-38 C. Protocol for “Candidate Projects” 39-42 as replacements to listed mitigative transportation projects Attachment A D. CIP Revenue Discussion 43-44 2 Executive Summary 1) Overview This Fort Ord Reuse Authority (FORA) Capital Improvement Program (CIP) is responsive to the capital improvement obligations defined under the Fort Ord Base Reuse Plan (BRP) as adopted by the FORA Board in June 1997. The BRP carries a series of mitigative project obligations defined in Appendix B of that plan as the Public Facilities Implementation Plan (PFIP). The PFIP, which serves as the baseline CIP for the reuse plan, is to be re-visited annually by the FORA Board to assure that required projects are implemented in a timely way to meet development needs. The PFIP was developed as a four-phase program spanning a twenty-year development horizon (1996-2015) and was based upon the best at-the-time forecasts of development patterns anticipated in concert with market absorption schedules for the area. -

Proposition 12 Allocation Balance Report
Proposition 12 Allocation Balance Report as of July 29, 2019 Public Resources Code 5096.310 Net Available for Enacted Bond Section Department/Program Appropriation* Appropriations** Proposed Balance §5096.310(a) Department of Parks and Recreation : Capital Outlay Projects 493,065,557 495,999,175 0 -2,933,618 (b) §5096.310(b) Department of Parks and Recreation : Stewardship Projects 17,661,973 17,661,973 0 0 §5096.310(c) Department of Parks and Recreation : Volunteers Projects 3,924,661 3,924,661 0 0 §5096.310(d) Department of Parks and Recreation : Locally-operated State Park Unit Grants 19,587,303 19,587,303 0 0 §5096.310(e) Department of Parks and Recreation : Office of Historic Preservation Grants 9,793,651 9,793,651 0 0 §5096.310(f) Department of Parks and Recreation : Per Capita Grants 379,854,866 379,828,335 0 26,531 §5096.310(g) Department of Parks and Recreation : Roberti-Z’Berg-Harris Grants 195,800,694 195,791,658 0 9,036 §5096.310(h) Department of Parks and Recreation : Riparian/Riverine Grants 9,789,935 9,789,483 0 452 §5096.310(i) Department of Parks and Recreation : Trail Grants 9,789,936 9,789,485 0 451 §5096.310(j) Department of Parks and Recreation : Murray-Hayden Grants 97,900,346 97,442,392 0 457,954 §5096.310(k) California Conservation Corps : State Projects 2,466,163 2,421,482 0 44,681 §5096.310(l) Department of Parks and Recreation : Zoos, Centers, Soccer Grants 84,682,916 84,679,008 0 3,908 §5096.310(m) Wildlife Conservation Board 261,632,536 262,612,879 0 -980,343 (a) §5096.310(n) California Tahoe Conservancy 49,264,259 -

Kenai National Wildlife Refuge Species List, Version 2018-07-24
Kenai National Wildlife Refuge Species List, version 2018-07-24 Kenai National Wildlife Refuge biology staff July 24, 2018 2 Cover image: map of 16,213 georeferenced occurrence records included in the checklist. Contents Contents 3 Introduction 5 Purpose............................................................ 5 About the list......................................................... 5 Acknowledgments....................................................... 5 Native species 7 Vertebrates .......................................................... 7 Invertebrates ......................................................... 55 Vascular Plants........................................................ 91 Bryophytes ..........................................................164 Other Plants .........................................................171 Chromista...........................................................171 Fungi .............................................................173 Protozoans ..........................................................186 Non-native species 187 Vertebrates ..........................................................187 Invertebrates .........................................................187 Vascular Plants........................................................190 Extirpated species 207 Vertebrates ..........................................................207 Vascular Plants........................................................207 Change log 211 References 213 Index 215 3 Introduction Purpose to avoid implying -

Arctic National Wildlife Refuge Volume 2
Appendix F Species List Appendix F: Species List F. Species List F.1 Lists The following list and three tables denote the bird, mammal, fish, and plant species known to occur in Arctic National Wildlife Refuge (Arctic Refuge, Refuge). F.1.1 Birds of Arctic Refuge A total of 201 bird species have been recorded on Arctic Refuge. This list describes their status and abundance. Many birds migrate outside of the Refuge in the winter, so unless otherwise noted, the information is for spring, summer, or fall. Bird names and taxonomic classification follow American Ornithologists' Union (1998). F.1.1.1 Definitions of classifications used Regions of the Refuge . Coastal Plain – The area between the coast and the Brooks Range. This area is sometimes split into coastal areas (lagoons, barrier islands, and Beaufort Sea) and inland areas (uplands near the foothills of the Brooks Range). Brooks Range – The mountains, valleys, and foothills north and south of the Continental Divide. South Side – The foothills, taiga, and boreal forest south of the Brooks Range. Status . Permanent Resident – Present throughout the year and breeds in the area. Summer Resident – Only present from May to September. Migrant – Travels through on the way to wintering or breeding areas. Breeder – Documented as a breeding species. Visitor – Present as a non-breeding species. * – Not documented. Abundance . Abundant – Very numerous in suitable habitats. Common – Very likely to be seen or heard in suitable habitats. Fairly Common – Numerous but not always present in suitable habitats. Uncommon – Occurs regularly but not always observed because of lower abundance or secretive behaviors. -

The Vascular Plants of British Columbia Part 1 - Gymnosperms and Dicotyledons (Aceraceae Through Cucurbitaceae)
The Vascular Plants of British Columbia Part 1 - Gymnosperms and Dicotyledons (Aceraceae through Cucurbitaceae) by George W. Douglas1, Gerald B. Straley2 and Del Meidinger3 1 George Douglas 2 Gerald Straley 3 Del Meidinger 6200 North Road Botanical Garden Research Branch R.R.#2 University of British Columbia B.C. Ministry of Forests Duncan, B.C. V9L 1N9 6501 S.W. Marine Drive 31 Bastion Square Vancouver, B.C. V6T 1Z4 Victoria, B. C. V8W 3E7 April 1989 Ministry of Forests THE VASCULAR PLANTS OF BRITISH COLUMBIA Part 1 - Gymnosperms and Dicotyledons (Aceraceae through Cucurbitaceae) Contributors: Dr. G.W. Douglas, Douglas Ecological Consultants Ltd., Duncan, B.C. — Aceraceae through Betulaceae Brassicaceae (except Arabis, Cardamine and Rorippa) through Cucurbitaceae. Mr. D. Meidinger, Research Branch, B.C. Ministry of Forests, Victoria, B.C. — Gymnosperms. Dr. G.B. Straley, Botanical Garden, University of B.C., Vancouver, B.C. — Boraginaceae, Arabis and Rorippa. With the cooperation of the Royal British Columbia Museum and the Botanical University of British Columbia. ACKNOWLEDGEMENTS We are grateful to Dr. G.A. Allen for providing valuable suggestions during the initial stages of the project. Thanks are also due to Drs. G.A. Allen, A. Ceska and F. Ganders for reviewing taxonomically difficult groups. Mrs. O. Ceska reviewed the final draft of Part 1. Mr. G. Mulligan kindly searched the DAO herbarium and provided information on Brassicaceae. Dr. G. Argus helped with records from CAN. Louise Gronmyr and Jean Stringer kindly typed most of the contributions and helped in many ways in the production of the final manuscript which was typeset by Beth Collins. -

Phylogenies and Secondary Chemistry in Arnica (Asteraceae)
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 392 Phylogenies and Secondary Chemistry in Arnica (Asteraceae) CATARINA EKENÄS ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 UPPSALA ISBN 978-91-554-7092-0 2008 urn:nbn:se:uu:diva-8459 !"# $ % !& '((" !()(( * * * + , - . , / , '((", + 0 1# 2, # , 34', 56 , , 70 46"84!855&86(4'8(, - 1# 2 . * 9 10-2 . * . # 9 , * * 1 ! " #! !$ 2 1 2 .8 # * * :# 77 1%&'(2 . !6 '3, + . .8 ) / , ; < * . * ** # , * * * , 09 * . # * * 33 * != , 0- # 9 * * 1, , * 2 . * , 0 * * * * * . * , $ * 0- * % # , # 8 * * * * * * $8> # . * * !' , * * . ** , ? . 0- , +,- # # 7-0 -0 :+' 9 +# $8> ./0) . ) 1 ) 2 * 3) ) .456(7 ) , @ / '((" 700 !=5!8='!& 70 46"84!855&86(4'8( ) ))) 8"&54 1 );; ,/,; A B ) ))) 8"&542 List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals: I Ekenäs, C., B. G. Baldwin, and K. Andreasen. 2007. A molecular phylogenetic -

Klondike Gold Rush National Historical Park Natural Resource Condition Assessment
National Park Service U.S. Department of the Interior Natural Resource Program Center Klondike Gold Rush National Historical Park Natural Resource Condition Assessment Natural Resource Report NPS/NRPC/WRD/NRR—2011/288 ON THE COVER. Taiya River Valley Photograph by: Barry Drazkowski, August 2009 Klondike Gold Rush National Historical Park Natural Resource Condition Assessment Natural Resource Report NPS/NRPC/WRD/NRR—2011/288 Barry Drazkowski Andrew Robertson Greta Bernatz GeoSpatial Services Saint Mary’s University of Minnesota 700 Terrace Heights, Box #7 Winona, Minnesota 55987 This report was prepared under Task Agreement J8W07080025 between the National Park Service and Saint Mary’s University of Minnesota, through the Pacific Northwest Cooperative Ecosystem Studies Unit. January 2011 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado i The National Park Service, Natural Resource Program Center publishes a range of reports that address natural resource topics of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate high-priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. Views, statements, findings, conclusions, recommendations, and data in this report do not necessarily reflect views and policies of the National Park Service, U.S. -

Arnica Lessingii Ssp
ARNICA LESSINGII SSP. NORBERGII IN THE PORTAGE AREA: A SENSITIVE SPECIES SURVEY A Report by Michael Duffy Alaska Natural Heritage Program ENVIRONMENT AND NATURAL RESOURCES INSTITUTE UNIVERSITY OF ALASKA ANCHORAGE 707 A Street, Anchorage, AK 99501 ARNICA LESSINGII SSP. NORBERGII IN THE PORTAGE AREA: A SENSITIVE SPECIES SURVEY A Report by Michael Duffy Alaska Natural Heritage Program ENVIRONMENT AND NATURAL RESOURCES INSTITUTE UNIVERSITY OF ALASKA ANCHORAGE 707 A Street, Anchorage, AK 99501 Submitted to: HDR ENGINEERING, INC. 2525 C Street Suite 305 Anchorage AK 99503-2689 October 21, 1994 Alaska Natural Heritage Program ENVIRONMENT AND NATURAL RESOURCES INSTITUTE UNIVERSITY OF ALASKA ANCHORAGE 707 A Street, Anchorage, AK 99501 ⋅ (907) 279-4523 ⋅ FAX (907) 276-6847 Dr. Douglas A. Segar, Director Dr. David C. Duffy, Program Manager UAA IS AN EO/AA EMPLOYER AND EDUCATIONAL INSTITUTION ______________________________________________________________________________ ACKNOWLEDGEMENTS ______________________________________________________________________________ I'd like to thank Connie Hubbard and Bill Queitzsch of Chugach National Forest and Anne Leggett of HDR, Inc. for participating in the field surveys; their botanical skills helped locate many of the arnica populations. Thanks also to Rob Lipkin, of The Alaska Natural Heritage Program, Dr. David Murray and the staff at the University of Alaska Fairbanks Museum Herbarium, and Mary Stensvold of Tongass National Forest. i TABLE OF CONTENTS ______________________________________________________________________________ -
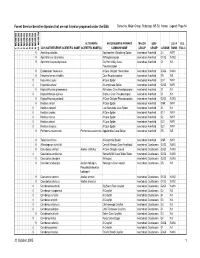
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

Kachemak Bay Research Reserve: a Unit of the National Estuarine Research Reserve System
Kachemak Bay Ecological Characterization A Site Profile of the Kachemak Bay Research Reserve: A Unit of the National Estuarine Research Reserve System Compiled by Carmen Field and Coowe Walker Kachemak Bay Research Reserve Homer, Alaska Published by the Kachemak Bay Research Reserve Homer, Alaska 2003 Kachemak Bay Research Reserve Site Profile Contents Section Page Number About this document………………………………………………………………………………………………………… .4 Acknowledgements…………………………………………………………………………………………………………… 4 Introduction to the Reserve ……………………………………………………………………………………………..5 Physical Environment Climate…………………………………………………………………………………………………… 7 Ocean and Coasts…………………………………………………………………………………..11 Geomorphology and Soils……………………………………………………………………...17 Hydrology and Water Quality………………………………………………………………. 23 Marine Environment Introduction to Marine Environment……………………………………………………. 27 Intertidal Overview………………………………………………………………………………. 30 Tidal Salt Marshes………………………………………………………………………………….32 Mudflats and Beaches………………………………………………………………………… ….37 Sand, Gravel and Cobble Beaches………………………………………………………. .40 Rocky Intertidal……………………………………………………………………………………. 43 Eelgrass Beds………………………………………………………………………………………… 46 Subtidal Overview………………………………………………………………………………… 49 Midwater Communities…………………………………………………………………………. 51 Shell debris communities…………………………………………………………………….. 53 Subtidal soft bottom communities………………………………………………………. 54 Kelp Forests…………………………………………………….…………………………………….59 Terrestrial Environment…………………………………………………………………………………………………. 61 Human Dimension Overview………………………………………………………………………………………………. -

Units by Classification
CALIFORNIA STATE PARKS UNITS BY CLASSIFICATION CULTURAL PRESERVES (CP): Marina SB (Marina Dunes NP) McGrath SB (Santa Clara Estuary NP) Carmel River SB (Ohlone Coastal CP) Montaña de Oro SP (Morro Dunes NP) Cuyamaca Rancho SP (Ah-Ha-Kwe-Ah-Mac/Stone- Morro Bay SP (Heron Rookery NP, Morro Estuary wall Mine CP, Cuish-Cuish CP, Kumeyaay Soapstone NP, Morro Rock NP) CP, Pilcha CP) Mount San Jacinto SP (Hidden Divide NP) Hungry Valley SVRA (Freeman Canyon CP, Gorman Natural Bridges SB (Monarch Butterfly NP, Moore CP, Tataviam CP) Creek Wetland NP) Millerton Lake SRA (Kechaye CP) Palomar Mountain SP (Doane Valley NP) Mount Diablo SP (Civilian Conservation Corps CP) Pescadero SB (Pescadero Marsh NP) Ocotillo Wells SVRA (Barrel Springs CP) Pismo SB (Pismo Dunes NP) San Simeon SP (Pâ-nu CP) Point Mugu SP (La Jolla Valley NP) Wilder Ranch SP (Wilder Dairy CP) Providence Mountains SRA (Mitchell Caverns NP) Red Rock Canyon SP (Hagen Canyon NP, Red Cliffs NATURAL PRESERVES (NP): NP) Salinas River SB (Salinas River Dunes NP, Salinas Anderson Marsh SHP (Anderson Marsh NP) River Mouth NP) Benicia SRA (Southampton Wetland NP) San Onofre SB (Trestles Wetlands NP) Big Basin Redwoods SP (Theodore J. Hoover NP) San Simeon SP (San Simeon NP, Santa Rosa Creek Border Field SP (Tijuana Estuary NP) NP) Burton Creek SP (Antone Meadows NP, Burton Silver Strand SB (Silver Strand NP) Creek NP) Sugar Pine Point SP (Edwin L. Z’berg NP) Calaveras Big Trees SP (Calaveras South Grove Sunset SB (Sunset Wetlands NP) NP) Torrey Pines SR (Ellen Browning Scripps NP, Los Carmel River SB (Carmel River Lagoon and Wet- Peñasquitos Marsh NP) land NP) Van Damme SP (Van Damme Pygmy Forest NP) Castle Rock SP (San Lorenzo Headwaters NP) Wilder Ranch SP (Wilder Beach NP) Chino Hills SP (Water Canyon NP) Woodson Bridge SRA (Woodson Bridge NP) Cuyamaca Rancho SP (Cuyamaca Meadow NP) Zmudowski SB (Pajaro River Mouth NP) Folsom Lake SRA (Anderson Island NP, Mormon Island Wetlands NP) REGIONAL INDIAN MUSEUMS: Harry A. -

Unit-Support Orglist 6-11
California State Parks Units with Supporting Organization (501(c) (3)) (updated 6/5/14) 1 Admiral William Stanley SRA (Richardson Grove Interpretive Association) 2 Ahjumawi Lava Springs SP 3 Albany State Reserve 4 Anderson Marsh SHP (Anderson Marsh Interpretive Association) 5 Andrew Molera SP (Big Sur Natural History Association) 6 Angel Island SP (Angel Island Conservancy) (Angel Island Immigration Station Foundation) 7 Annadel SP (Valley of the Moon Natural History Association) 8 Ano Nuevo State Park (Coastside Parks Association) 9 Año Nuevo SNR (Coastside Parks Association) 10 Antelope Valley California Poppy Reserve (Poppy Reserve/Mojave Desert Interpretive Association) 11 Antelope Valley Indian Museum (Friends of the Antelope Valley Indian Museum) 12 Anza-Borrego Desert SP (Anza-Borrego Foundation & Institue) 13 Armstrong Redwoods SNR (Stewards of the Coast and Redwoods) 14 Arthur B. Ripley Desert Woodland SP (Poppy Reserve Mojave Desert Interpretive Association) 15 Asilomar State Beach 16 Auburn SRA 17 Austin Creek SRA (Stewards of the Coast and Redwoods) 18 Azalea SNR 19 Bale Grist Mill SHP (Napa Valley Natural History Association) 20 Bean Hollow SB (Coastside Parks Association) 21 Benbow Lake SRA (Richardson Grove Interpretive Association) 22 Benicia Capitol SHP (Benicia State Parks Association) 23 Benicia SRA (Benicia State Parks Association) 24 Bethany Reservoir SRA 25 Bidwell Mansion SHP (Bidwell Mansion Association) 26 Bidwell-Sacramento River SP Big Basin Redwoods SP (Mountain Parks Foundation; Rancho del Oso section-Waddell Creek Assn.) 27 28 Bodie SHP (Bodie Foundation) 29 Bolsa Chica SB Amigos de Bolsa Chica Border Field SP (Tijuana River National Estuary RP-Southwest Wetlands Interpretive Association, 30 Friends of San Diego Wildlife Refuges, inc.) 31 Bothe-Napa Valley SP (Napa Valley State Parks Association) 32 Brannan Island SRA 33 Burleigh H.