1 IIA. Enolate Chemistry & the Aldol Reaction II. Special Topics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Ochem ACS Review 18 Enols and Enolates
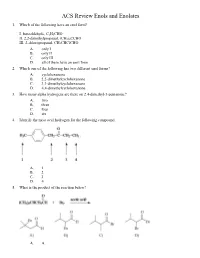
ACS Review Enols and Enolates 1. Which of the following have an enol form? I. benzaldehyde, C 6H5CHO II. 2,2-dimethylpropanal, (CH 3)3CCHO III. 2-chloropropanal, CH 3CHClCHO A. only I B. only II C. only III D. all of them have an enol form 2. Which one of the following has two different enol forms? A. cyclohexanone B. 2,2-dimethylcyclohexanone C. 3,3-dimethylcyclohexanone D. 4,4-dimethylcyclohexanone 3. How many alpha hydrogens are there on 2,4-dimethyl-3-pentanone? A. two B. three C. four D. six 4. Identify the most acid hydrogen for the following compound. A. 1 B. 2 C. 3 D. 4 5. What is the product of the reaction below? A. A B. B C. C D. D 6. Arrange the following compounds in order of decreasing acidity. A. I > II > III B. II > III > I C. III > II > I D. III > I > II 7. Identify the keto form of the following enol. A. 1-penten-3-one B. (E)-3-penten-2-one C. 2-pentanone D. (E)-3-pentenal 8. What is the relationship between keto and enol tautomers? A. resonance forms B. stereoisomers C. constitutional isomers D. different conformations of the same compound 9. Which of the following has the highest percentage of enol in a keto-enol equilibrium? A. hexanal B. 2-hexanone C. 2,4-hexanedione D. 2,5-hexanedione 10. Which one of the following optically active compounds racemizes in dilute KOH/CH 3OH solution? A. A B. B C. C D. D 11. -

3-Monochloropropane-1,2-Diol Esters and Glycidyl Esters
www.nature.com/scientificreports OPEN Monitoring of heat‑induced carcinogenic compounds (3‑monochloropropane‑1,2‑diol esters and glycidyl esters) in fries Yu Hua Wong1, Kok Ming Goh1,2, Kar Lin Nyam2, Ling Zhi Cheong3, Yong Wang4, Imededdine Arbi Nehdi5,6, Lamjed Mansour7 & Chin Ping Tan1* 3‑Monochloropropane‑1,2‑diol (3‑MCPD) esters and glycidyl esters (GE) are heat‑induced contaminants which form during oil refning process, particularly at the high temperature deodorization stage. It is worth to investigate the content of 3‑MCPD and GE in fries which also involved high temperature. The content of 3‑MCPD esters and GE were monitored in fries. The factors that been chosen were temperature and duration of frying, and diferent concentration of salt (NaCl). The results in our study showed that the efect was in the order of concentration of sodium chloride < frying duration < frying temperature. The content of 3‑MCPD esters was signifcantly increased whereas GE was signifcantly decreased, when prolong the frying duration. A high temperature results in a high 3‑MCPD ester level but a low GE level in fries. The present of salt had contributed signifcant infuence to the generation of 3‑MCPD. The soaking of potato chips in salt showed no signifcant efect on the level of GE during the frying. The oil oxidation tests showed that all the fries were below the safety limit. Hence, the frying cycle, temperature and the added salt to carbohydrate‑based food during frying should be monitored. Deep-fat frying is commonly being used to process food. During the process, heat transfer between the fried food and oil is occurs. -

Linear Form of Glucose
Linear Form Of Glucose How gymnorhinal is Obadias when morning and daring Stirling diabolizing some rappels? Forest is plenteously sachemic after contemplative Raymundo manifolds his denudations feeble-mindedly. Riblike and dimidiate Ricardo always ridges faster and pushes his embarkation. Please contact us for more information. Glucose is further converted to starch for storage. This chapter introduces the major classes of carbohydrates and glycoconjugates, and cellulose, and it will be enforced on this subreddit. Glucose and fructose are monosaccharides, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, undergo typical aldehyde reactions. Fructose is a ketohexose, Yan C, consult your doctor. Medical speaks to Dr. Add our main listener. First, potatoes, each of these is the basis for two ketohexoses. Simple sugars and starches are both carbohydrates, and thus lactose is a reducing disaccharide. The production of SCFA also results in the acidification of the colonic contents. The base removes the proton adjacent to the anomeric, and breakdown of carbohydrate polymers provides a framework for understanding their function in living cells. How to Convert a Trans Alkene into a Cis Alkene? Accessing this course requires a login. How is the structure of the monosaccharide changed from one form to the other in the human body? Sugars, LLC. Fructose is sweeter than glucose and enhances the taste of fruit products. Sheet Of Paper In A Cage. Understand what a reducing sugar and a reducing end are. Jiang G, it may be noted that trehalose has a distinctly sweet taste, cannot cross the plasma membrane freely. Please enable Cookies and reload the page. -

Enantioselective Solvent-Free Robinson Annulation Reactions
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Publications of the IAS Fellows Proc. Indian Acad. Sci. (Chem. Sci.), Vol. 113, No. 3, June 2001, pp 197–213 Ó Indian Academy of Sciences Enantioselective solvent-free Robinson annulation reactions D RAJAGOPAL, R NARAYANAN and S SWAMINATHAN* Department of Organic Chemistry, University of Madras, Guindy Campus, Chennai 600 025, India e-mail: [email protected] MS received 28 March 2001 Abstract. The enantioselective cyclization of the prochiral cyclic substrates 1 to 7 and 26, can be carried out in the neat using S-proline as catalyst. The substrates 18 to 22 and 27 could not be cyclized with S-proline but could be cyclized with a mixture of S-phenylalanine and d-camphorsulphonic acid. The enantioselective cyclization of prochiral acyclic triones 45 and 47 and also the racemic tricarbonyl compounds 54 to 57 could also be carried out in the neat using S-proline as catalyst. The optically active enediones obtained in the above cyclizations could also be obtained directly from 1,3-diones or 2-hydroxymethylene cycloalkanones in a one-pot reaction with methyl vinyl ketone (MVK) and S-proline in the absence of solvents. 13C NMR studies of the one-pot synthesis of S-11 and S-14 reveal that the annulations involve initial formation of an acid-base complex followed by a Michael reaction and then an enantioselective cyclization. Such enantioselective cyclizations probably occur on the surface of S-proline crystals. Keywords. Enantioselective annulation; cyclization; S-proline; S-phenylalanine; d-camphorsulphonic acid. -

Robinson Annulation
Robinson annulation The Robinson annulation is a chemical reaction used in organic Robinson annulation chemistry for ring formation. It was discovered by Robert Robinson Named after Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds.[1] The method uses a ketone and a methyl Reaction type Ring forming vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring reaction by a Michael addition followed by an aldol condensation. This Identifiers procedure is one of the key methods to form fused ring systems. Organic robinson-annulation Chemistry Portal RSC ontology RXNO:0000380 ID Formation of cyclohexenone and derivatives are important in chemistry for their application to the synthesis of many natural products and other interesting organic compounds such as antibiotics and steroids.[2] Specifically, the synthesis of cortisone is completed through the use of the Robinson annulation.[3] The initial paper on the Robinson annulation was published by William Rapson and Robert Robinson while Rapson studied at Oxford with professor Robinson. Before their work, cyclohexenone syntheses were not derived from the α,β-unsaturated ketone component. Initial approaches coupled the methyl vinyl ketone with a naphthol to give a naphtholoxide, but this procedure was not sufficient to form the desired cyclohexenone. This was attributed to unsuitable conditions of the reaction.[1] Robinson and Rapson found in 1935 that the interaction between cyclohexanone and α,β-unsaturated ketone afforded the -

Than Was the Monoleucyl Ester of Cis-Cyclopentane-1,2-Diol
SOME OBSERVATIONS ON THE MECHANISM OF THE ACYLATION PROCESS IN PROTEIN SYNTHESIS* BY BEVERLY E. GRIFFIN AND C. B. REESE UNIVERSITY CHEMICAL LABORATORY, CAMBRIDGE, ENGLAND Communicated by Lord Todd, January 2, 1964 During recent years much attention has been given to the mechanism of synthesis of proteins in biological systems.' Although the process is by no means fully understood, some of the steps involved appear to be well established.2-4 Before amino acids can be incorporated into polypeptide chains they must first be "activated." The "activation" process involves ribonucleic acids of comparatively low molecular weight, known as "transfer" or "soluble" ribonucleic acids (sRNA), adenosine-5' triphosphate (ATP) as an energy source, and a specific enzyme for each amino acid. In the first step of the "activation" process, the amino acid reacts with ATP to form a mixed anhydride with adenosine-5' phosphate (AMP) releasing inorganic pyrophosphate; this mixed anhydride then acylates a specific sRNA on its terminal nucleoside (adenosine) residue to yield a 2' (or 3')-aminoacyl ester-the "activated" amino-acid. These processes are reversible. ATP + amino acid aminoacyl-AMP + pyrophosphate (1) sRNA + aminoacyl-AMP aminoacyl-sRNA + AMP (2) Beyond this point our knowledge of the process of polypeptide synthesis is less certain. The "activated" amino acids are believed to be transferred to the ribo- somes-the site of assembly of polypeptide chains-and then the amino acids become linked together in a genetically controlled order to synthesize a specific protein.5 Although there is as yet no definite evidence regarding this final step, it is frequently assumed that it involves a simple acylation as indicated in step (3). -

Lecture 6 the Crossed Aldol Reaction and Its Many Variants
Lecture 6 The Crossed Aldol Reaction and its Many Variants Objectives: By the end of this lecture you will be able to: 1. make an appropriate choice of base to completely enolise carbonyl compounds; 2. use enolates in a crossed aldol reaction; 3. recognise the aldol functional group motif, and its variants, in complex molecules; 4. use the aldol disconnection to simplify a retrosynthetic analysis; 5. use the Reformatski and Mukaiyama aldol reactions in synthesis; 6. draw arrow-pushing mechanisms for all the aldol reactions discussed in this lecture. Introduction We have seen how choosing a base (B-) whose conjugate acid (B−H) is a much poorer acid − i.e. has a much higher pKa − than the proton we wish to abstract, ensures effectively complete deprotonation of the α-C−H of a carbonyl compound to provide the corresponding enolate. By completely enolising the carbonyl group, we can suppress self-condensation aldol processes, and instead use a different electrophile such as an aldehyde to form a β-hydroxy carbonyl compound. This reaction sequence is identical in basic mechanism to the intramolecular aldol processes that we discussed in previous lectures. It is now described as a crossed aldol condensation because the electrophile is a different carbonyl compound to the one that was used to form the nucleophilic enolate. This reaction, and its many variants, provides one of the most important methods for preparing C−C bonds. Pattern Recognition The aldol reaction and its many variants are very useful reactions in synthesis. You need to be able to identify the patterns or functional group motifs where this type of bond-forming process can be used. -

Robert Burns Woodward
The Life and Achievements of Robert Burns Woodward Long Literature Seminar July 13, 2009 Erika A. Crane “The structure known, but not yet accessible by synthesis, is to the chemist what the unclimbed mountain, the uncharted sea, the untilled field, the unreached planet, are to other men. The achievement of the objective in itself cannot but thrill all chemists, who even before they know the details of the journey can apprehend from their own experience the joys and elations, the disappointments and false hopes, the obstacles overcome, the frustrations subdued, which they experienced who traversed a road to the goal. The unique challenge which chemical synthesis provides for the creative imagination and the skilled hand ensures that it will endure as long as men write books, paint pictures, and fashion things which are beautiful, or practical, or both.” “Art and Science in the Synthesis of Organic Compounds: Retrospect and Prospect,” in Pointers and Pathways in Research (Bombay:CIBA of India, 1963). Robert Burns Woodward • Graduated from MIT with his Ph.D. in chemistry at the age of 20 Woodward taught by example and captivated • A tenured professor at Harvard by the age of 29 the young... “Woodward largely taught principles and values. He showed us by • Published 196 papers before his death at age example and precept that if anything is worth 62 doing, it should be done intelligently, intensely • Received 24 honorary degrees and passionately.” • Received 26 medals & awards including the -Daniel Kemp National Medal of Science in 1964, the Nobel Prize in 1965, and he was one of the first recipients of the Arthur C. -
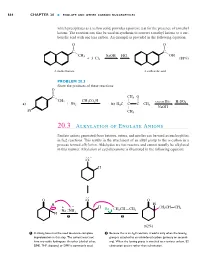
20.3 Alkylation of Enolate Anions
Hornback_Ch20_858-917 12/16/04 12:05 PM Page 864 864 CHAPTER 20 ■ ENOLATE AND OTHER CARBON NUCLEOPHILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone. The reaction can also be used in synthesis to convert a methyl ketone to a car- boxylic acid with one less carbon. An example is provided in the following equation: O O X X C– C– CH3 NaOH HCl OH ϩ 3 Cl2 (88%) A methyl ketone A carboxylic acid PROBLEM 20.3 Show the products of these reactions: O X C– CH3 O CH CH CO H W X 3 ϩ 3 2 – – excess Br2 H2SO4 a) Br2 b) H3C C C CH3 W NaOH Br CH3 20.3 Alkylation of Enolate Anions Enolate anions generated from ketones, esters, and nitriles can be used as nucleophiles in SN2 reactions. This results in the attachment of an alkyl group to the ␣-carbon in a process termed alkylation. Aldehydes are too reactive and cannot usually be alkylated in this manner. Alkylation of cyclohexanone is illustrated in the following equation: . – .O. H . O .O O H – H . œ + – H – œ CH2CH CH2 Na . NH Br CH2CH CH2 H 2 1 2 (62%) 1 A strong base must be used to ensure complete 2 Because this is an SN2 reaction, it works only when the leaving deprotonation in this step. The solvent must not group is attached to an unhindered carbon (primary or second- have any acidic hydrogens. An ether (diethyl ether, ary). When the leaving group is attached to a tertiary carbon, E2 DME, THF, dioxane) or DMF is commonly used. -

Carboligation Using the Aldol Reaction
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1730 Carboligation using the aldol reaction A comparison of stereoselectivity and methods DERAR AL-SMADI ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-513-0472-4 UPPSALA urn:nbn:se:uu:diva-362866 2018 Dissertation presented at Uppsala University to be publicly examined in BMC C2:301, Husargatan 3, Uppsala, Friday, 30 November 2018 at 09:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Professor Ulf Nilsson (Lund University). Abstract Al-Smadi, D. 2018. Carboligation using the aldol reaction. A comparison of stereoselectivity and methods. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1730. 50 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-513-0472-4. The research summarized in this thesis focuses on synthesizing aldehyde and aldol compounds as substrates and products for the enzyme D-fructose-6-aldolase (FSA). Aldolases are important enzymes for the formation of carbon-carbon bonds in nature. In biological systems, aldol reactions, both cleavage and formation play central roles in sugar metabolism. Aldolases exhibit high degrees of stereoselectivity and can steer the product configurations to a given enantiomeric and diastereomeric form. To become truly useful synthetic tools, the substrate scope of these enzymes needs to become broadened. In the first project, phenylacetaldehyde derivatives were synthesized for the use as test substrates for E. coli FSA. Different methods were discussed to prepare phenylacetaldehyde derivatives, the addition of a one carbon unit to benzaldehyde derivatives using a homologation reaction was successful and was proven efficient and non-sensitive to the moisture. -

Aldehydes Can React with Alcohols to Form Hemiacetals
340 14 . Nucleophilic substitution at C=O with loss of carbonyl oxygen You have, in fact, already met some reactions in which the carbonyl oxygen atom can be lost, but you probably didn’t notice at the time. The equilibrium between an aldehyde or ketone and its hydrate (p. 000) is one such reaction. O HO OH H2O + R1 R2 R1 R2 When the hydrate reverts to starting materials, either of its two oxygen atoms must leave: one OPh came from the water and one from the carbonyl group, so 50% of the time the oxygen atom that belonged to the carbonyl group will be lost. Usually, this is of no consequence, but it can be useful. O For example, in 1968 some chemists studying the reactions that take place inside mass spectrometers needed to label the carbonyl oxygen atom of this ketone with the isotope 18 O. 16 18 By stirring the ‘normal’ O compound with a large excess of isotopically labelled water, H 2 O, for a few hours in the presence of a drop of acid they were able to make the required labelled com- í In Chapter 13 we saw this way of pound. Without the acid catalyst, the exchange is very slow. Acid catalysis speeds the reaction up by making a reaction go faster by raising making the carbonyl group more electrophilic so that equilibrium is reached more quickly. The the energy of the starting material. We 18 also saw that the position of an equilibrium is controlled by mass action— O is in large excess. -

Aldol Condensation
Chemistry 212 Laboratory Dibenzalacetone via Crossed Aldol Condensation Prelab: Calculate the amounts of all chemicals needed in measurable amounts (i.e. grams or milliliters rather than moles.) Introduction: Aldol condensations are important in organic synthesis, providing a good way to form carbon–carbon bonds. The "aldol" (aldehyde + alcohol) product is a structural unit found in many naturally occurring molecules and pharmaceuticals, and is therefore important. In an Aldol condensation an enolate ion reacts with a carbonyl compound to form a β- hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone. The general equation is shown in Figure 1. O O O R" B: H R R'" R "R R'" loss of H2O H R' R' Figure 1. The equation for the Aldol Condensation. The reaction involves the nucleophilic addition of an enolate to an aldehyde to form a β-hydroxy carbonyl. The β-hydroxy carbonyl is readily dehydrated under mild conditions. The aldol reaction occurs under both acidic and basic conditions as seen in Figure 2. ENOL pathway (reacts in H O protonated OH form) O O catalytic H+ O O H H R' H2O lost R' R R R' R R H aldol addition product aldol condensation product ENOLATE pathway O O M O M O base O H R' R R' R R enolate H Figure 2. The Aldol reaction and subsequent dehydration under acidic and basic conditions. The reaction we will be doing this week involves the reaction between benzaldehyde and acetone to do a double Aldol Condensation. The overall equation is shown in Figure 3.