Supraspecific Taxonomy in the Vertiginidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Pulmonata: Vertiginidae) and Strobilops
Records of the Hawaii Biological Survey for 2012. Edited by Neal L. Evenhuis & Lucius G. Eldredge. Bishop Museum Occasional Papers 114: 39 –42 (2013) Hawaiian land snail records : Lyropupa cookei Clench , 1952 (Pulmonata : Vertiginidae ) and Strobilops aeneus Pilsbry , 1926 (Pulmonata : Strobilopsidae ) CARl C. C HRiSTeNSeN Bishop Museum, 1525 Bernice Street, Honolulu, Hawai‘i 96817-2704, USA; email: [email protected] This note clarifies the status of two taxa of land snails that have been reported to occur in the Hawaiian islands. Lyropupa cookei Clench, 1952, is shown to be a synonym of Lyropupa anceyana Cooke & Pilsbry in Pilsbry & Cooke, 1920. The sole Hawaiian record for the North American Strobilops aeneus Pilsbry, 1926, is almost certainly based on a mislabeled specimen, and accordingly this species should be removed from the Hawaiian faunal list. Lyropupa cookei Clench , 1952 Lyropupa Pilsbry, 1900, is a genus of pupilloid land snails endemic to the Hawaiian islands. in their monograph of the genus, Pilsbry & Cooke (1920 in 1918–1920: 253–254, pl. 26, figs. 3, 6) published a description of “ Lyropupa anceyana C. & P., n. sp.,” based on specimens from ola‘a on the island of Hawai‘i held in the collections of Bishop Museum and the Academy of Natural Sciences of Philadelphia. They stated that their new species had previously been misidentified by Ancey (1904:124) as Lyropupa lyrata (Gould, 1843) . Several pages earlier, in their systematic treatment of that species, Pilsbry & Cooke (1918–1920: 235) had also set forth their conclusion that Ancey had misidenti - fied Gould’s species and stated that in fact Ancey’s “description of lyrata was based on specimens of an unnamed species for which the name L. -

Pu'u Wa'awa'a Biological Assessment
PU‘U WA‘AWA‘A BIOLOGICAL ASSESSMENT PU‘U WA‘AWA‘A, NORTH KONA, HAWAII Prepared by: Jon G. Giffin Forestry & Wildlife Manager August 2003 STATE OF HAWAII DEPARTMENT OF LAND AND NATURAL RESOURCES DIVISION OF FORESTRY AND WILDLIFE TABLE OF CONTENTS TITLE PAGE ................................................................................................................................. i TABLE OF CONTENTS ............................................................................................................. ii GENERAL SETTING...................................................................................................................1 Introduction..........................................................................................................................1 Land Use Practices...............................................................................................................1 Geology..................................................................................................................................3 Lava Flows............................................................................................................................5 Lava Tubes ...........................................................................................................................5 Cinder Cones ........................................................................................................................7 Soils .......................................................................................................................................9 -

Spatial Predictive Distribution Modelling of Madeira's Endemic
DEPARTAMENTO DE ZOOLOGIA FACULDADE DE CIÊNCIAS E TECNOLOGIA UNIVERSIDADE DE COIMBRA Spatial predictive distribution modelling of Madeira’s endemic land snail species Dinarte Nuno Freitas Teixeira 2009 REGIÃO AUTÓNOMA DA REPÚBLICA PORTUGUESA UNIÃO EUROPEIA MADEIRA FSE DEPARTAMENTO DE ZOOLOGIA FACULDADE DE CIÊNCIAS E TECNOLOGIA UNIVERSIDADE DE COIMBRA Spatial predictive distribution modelling of Madeira’s endemic land snail species Dissertação apresentada à Universidade de Coimbra para cumprimento dos requisitos necessários à obtenção do grau de Mestre em Ecologia, realizada sob a orientação científica do Professor Doutor José Paulo Sousa (Universidade de Coimbra) e do Professor Doutor José Manuel Jesus (Universidade da Madeira). Dinarte Nuno Freitas Teixeir a 2009 O presente trabalho foi financiado pelo Centro de Ciência e Tecnologia da Madeira (CITMA), através da bolsa de Mestrado FSE BM I/2008 – 531, ao abrigo do Programa Operacional de Valorização do Potencial Humano e Coesão Social da RAM (RUMOS). REGIÃO AUTÓNOMA DA MADEIRA REPÚBLICA PORTUGUESA UNIÃO EUROPEIA FSE À Susana AGRADECIMENTOS Esta tese é o resultado de um trabalho conjunto para o qual muitos contribuíram e aos quais desejo reconhecer e deixar o meu agradecimento. Ao professor Doutor José Paulo Sousa, meu orientador, pela indispensável ajuda, paciência e orientação científica. Ao professor Doutor José Manuel Jesus, meu orientador, pela amizade e apoio desde os primeiros momentos. Pelo seu empenho, conselhos transmitidos, chamadas à razão e orientação científica o meu muito obrigado. Ao Doutor Pedro Cardoso, meu orientador e a quem muito devo, pelo constante acompanhamento e disponibilidade, amizade e orientação científica. Por tudo o que me ensinou, pela motivação e animo que sempre me transmitiu, e, acima de tudo, pela manutenção da objectividade do trabalho. -

Molluscs of the Dürrenstein Wilderness Area
Molluscs of the Dürrenstein Wilderness Area S a b i n e F ISCHER & M i c h a e l D UDA Abstract: Research in the Dürrenstein Wilderness Area (DWA) in the southwest of Lower Austria is mainly concerned with the inventory of flora, fauna and habitats, interdisciplinary monitoring and studies on ecological disturbances and process dynamics. During a four-year qualitative study of non-marine molluscs, 96 sites within the DWA and nearby nature reserves were sampled in cooperation with the “Alpine Land Snails Working Group” located at the Natural History Museum of Vienna. Altogether, 84 taxa were recorded (72 land snails, 12 water snails and mussels) including four endemics and seven species listed in the Austrian Red List of Molluscs. A reference collection (empty shells) of molluscs, which is stored at the DWA administration, was created. This project was the first systematic survey of mollusc fauna in the DWA. Further sampling might provide additional information in the future, particularly for Hydrobiidae in springs and caves, where detailed analyses (e.g. anatomical and genetic) are needed. Key words: Wilderness Dürrenstein, Primeval forest, Benign neglect, Non-intervention management, Mollusca, Snails, Alpine endemics. Introduction manifold species living in the wilderness area – many of them “refugees”, whose natural habitats have almost In concordance with the IUCN guidelines, research is disappeared in today’s over-cultivated landscape. mandatory for category I wilderness areas. However, it may not disturb the natural habitats and communities of the nature reserve. Research in the Dürrenstein The Dürrenstein Wilderness Area Wilderness Area (DWA) focuses on providing invento- (DWA) ries of flora and fauna, on interdisciplinary monitoring The Dürrenstein Wilderness Area (DWA) was as well as on ecological disturbances and process dynamics. -

Euconulus Alderi Gray a Land Snail
Euconulus alderi, Page 1 Euconulus alderi Gray A land snail State Distribution Photo by Matthew Barthel and Jeffery C. Nekola Best Survey Period Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Status: State listed as Special Concern are smaller, have a more shiny luster, and a darker shell color. Also, the microscopic spiral lines on the base of Global and state ranks: G3Q/S2 the shell are stronger than the radial striations. This is reversed in E. fulvus (Nekola 1998). For more Family: Helicarionidae information on identifying land snails, see Burch and Jung (1988) pages 155-158 or Burch and Pearce (1990) Synonyms: none pages 211-218. Total range: The global range of Euconulus alderi Best survey time: Surveys for E. alderi are best includes Ireland, Sweden, the United Kingdom, and the performed after rain, when the soil and vegetation are United States. Within the U.S. it has been found in moist. During dry periods, a survey site can appear Iowa, Maine, Massachusetts, Michigan, Minnesota, and completely devoid of snails, while after a rain the same Wisconsin (Frest 1990, NatureServe 2007, Nekola site can be found to contain numerous individuals. 1998). This species was not known from North Temperatures should be warm enough that the ground is America until 1986 when it was discovered in Iowa and not frozen and there is no snow. Dry, hot periods during Wisconsin (Frest 1990, Nekola 1998). mid-summer should be avoided. The best time of day to survey is often in early morning when conditions are State distribution: E. -
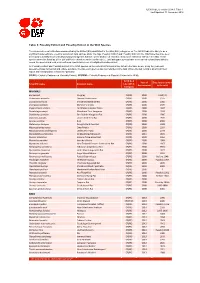
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2014.3: Table 9 Last Updated: 13 November 2014 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red Year of Date last recorded Scientific name Common name List (2014) Assessment in the wild Category MAMMALS Bos sauveli Kouprey CR(PE) 2008 1969/70 Crateromys australis Dinagat Crateromys CR(PE) 2008 1975 Crocidura trichura Christmas Island Shrew CR(PE) 2008 1985 Crocidura wimmeri -

Fauna of New Zealand Ko Te Aitanga Pepeke O Aotearoa
aua o ew eaa Ko te Aiaga eeke o Aoeaoa IEEAE SYSEMAICS AISOY GOU EESEAIES O ACAE ESEAC ema acae eseac ico Agicuue & Sciece Cee P O o 9 ico ew eaa K Cosy a M-C aiièe acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa EESEAIE O UIESIIES M Emeso eame o Eomoogy & Aima Ecoogy PO o ico Uiesiy ew eaa EESEAIE O MUSEUMS M ama aua Eiome eame Museum o ew eaa e aa ogaewa O o 7 Weigo ew eaa EESEAIE O OESEAS ISIUIOS awece CSIO iisio o Eomoogy GO o 17 Caea Ciy AC 1 Ausaia SEIES EIO AUA O EW EAA M C ua (ecease ue 199 acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 38 Naturalised terrestrial Stylommatophora (Mousca Gasooa Gay M ake acae eseac iae ag 317 amio ew eaa 4 Maaaki Whenua Ρ Ε S S ico Caeuy ew eaa 1999 Coyig © acae eseac ew eaa 1999 o a o is wok coee y coyig may e eouce o coie i ay om o y ay meas (gaic eecoic o mecaica icuig oocoyig ecoig aig iomaio eiea sysems o oewise wiou e wie emissio o e uise Caaoguig i uicaio AKE G Μ (Gay Micae 195— auase eesia Syommaooa (Mousca Gasooa / G Μ ake — ico Caeuy Maaaki Weua ess 1999 (aua o ew eaa ISS 111-533 ; o 3 IS -7-93-5 I ie 11 Seies UC 593(931 eae o uIicaio y e seies eio (a comee y eo Cosy usig comue-ase e ocessig ayou scaig a iig a acae eseac M Ae eseac Cee iae ag 917 Aucka ew eaa Māoi summay e y aco uaau Cosuas Weigo uise y Maaaki Weua ess acae eseac O o ico Caeuy Wesie //wwwmwessco/ ie y G i Weigo o coe eoceas eicuaum (ue a eigo oaa (owe (IIusao G M ake oucio o e coou Iaes was ue y e ew eaIa oey oa ue oeies eseac -

Les Mollusques Continentaux De La Région Nord-Pas-De-Calais Liste Des Espèces, Échantillonnage Et Base De Données
Université des Sciences et Technologies de Lille – U.F.R. de Biologie Année 2003 n°ordre Diplôme Supérieur de Recherche en Sciences Naturelles Présenté et soutenu publiquement par XAVIER CUCHERAT le 2 juillet 2003 Les Mollusques Continentaux de la Région Nord-Pas-de-Calais Liste des espèces, Échantillonnage et Base de Données Jury PR. M. DESCAMPS Université de Lille I Président DR. A. LEPRETRE Université de Lille I Rapporteur DR. S. DEMUYNCK Université de Lille I Examinateur DR. J. GODIN Université de Lille I Examinateur DR. J. PRYGIEL Agence de l’Eau Artois-Picardie Examinateur Université des Sciences et Technologies de Lille – U.F.R. de Biologie Année 2003 n°ordre Diplôme Supérieur de Recherche en Sciences Naturelles Présenté et soutenu publiquement par XAVIER CUCHERAT le 2 juillet 2003 Les Mollusques Continentaux de la Région Nord-Pas-de-Calais Liste des espèces, Échantillonnage et Base de Données Arion rufus (LINNAEUS 1758) Lymnaea stagnalis (LINNAEUS 1758) Adultes en parade nuptiale / Forêt Domaniale Adulte / dunes d’Erdeven / Erdeven de Mormal / Locquignol (Nord). 09/2001. (Morbihan). 05/2001. Taille des individus : 90 mm. Taille de l’individu : 60 mm. Photo : GUILLAUME EVANNO Photo : GUILLAUME EVANNO Malacolimax tenellus (O. F. MÜLLER 1774) Cepaea nemoralis nemoralis (LINNAEUS Adulte / Forêt Domaniale de Mormal / 1758) Locquignol (Nord). 09/2001. Adulte / Guebwiller (Haut-Rhin). 04/2003. Taille de l’individu : 35 mm. Taille de l’individu : 25mm. Photo : GUILLAUME EVANNO Photo : ALAIN LEPRETRE Jury PR. M. DESCAMPS Université de Lille I Président DR. A. LEPRETRE Université de Lille I Rapporteur DR. S. DEMUYNCK Université de Lille I Examinateur DR. -

Organismu Latviskie Nosaukumi (2)
Biosistēmu Terminoloģijas Centra Biļetens 1(1) (2017): 21–51 ISSN 2501-0336 (online) http://www.rpd-science.org/BTCB/V001/BTCB_1_4.pdf © “RPD Science” Citēšanai: BTCB, 2017. Organismu latviskie nosaukumi (2). Biosistēmu Terminoloģijas Centra Biļetens 1(1): 21–51 Organismu latviskie nosaukumi (2) Latvian names of organisms (2) Zinātniskais nosaukums Atbilstība Pēdējā Scientific name Equivalence pārbaude Last verification A Abies gmelinii Rupr. (1845) = Larix gmelinii (Rupr.) Rupr. var. gmelinii 17.12.2016. Abies ledebourii Rupr. (1845) = Larix gmelinii (Rupr.) Rupr. var. gmelinii 17.12.2016. Abies menziesii Mirb. (1825) = Pseudotsuga menziesii (Mirb.) Franco var. menziesii 17.12.2016. Acaciaceae = Fabaceae 27.12.2016. Acalitus brevitarsus (Fockeu, 1890) melnalkšņa maurērce 16.12.2016. Acalitus calycophthirus (Nalepa, 1891) bērzu pumpurērce 16.12.2016. Acalitus essigi (Hassan, 1928) aveņu pangērce 16.12.2016. Acalitus longisetosus (Nalepa, 1892) bērzu sārtā maurērce 16.12.2016. Acalitus phloeocoptes (Nalepa, 1890) plūmju stumbra pangērce 16.12.2016. Acalitus phyllereus (Nalepa, 1919) baltalkšņa maurērce 16.12.2016. Acalitus plicans (Nalepa, 1917) dižskābaržu maurērce 16.12.2016. Acalitus rudis (Canestrini, 1890) bērzu baltā maurērce 16.12.2016. Acalitus stenaspis (Nalepa, 1891) dižskābaržu lapmalērce 16.12.2016. Acalitus vaccinii (Keifer, 1939) melleņu pumpurērce 16.12.2016. Acanthinula aculeata (O. F. Müller, 1774) mazais dzeloņgliemezis 16.12.2016. Acanthinula spinifera Mousson, 1872 Spānijas dzeloņgliemezis 16.12.2016. Acanthocardia echinata (Linnaeus, 1758) dzelkņainā sirsniņgliemene 16.12.2016. Acanthochitona crinita (Pennant, 1777) zaļais bruņgliemis 16.12.2016. Aceria brevipunctatus (Nalepa, 1889) = Aceria campestricola (Frauenfeld, 1865) 16.12.2016. Aceria brevirostris (Nalepa, 1892) ziepenīšu pangērce 16.12.2016. Aceria brevitarsus (Fockeu, 1890) = Acalitus brevitarsus (Fockeu, 1890) 16.12.2016. -

Using the Jolly-Seber Model to Characterise Xerolenta Obvia (Gastropoda: Geomitridae) Population ISSN 2255-9582
Environmental and Experimental Biology (2020) 18: 83–94 Original Paper http://doi.org/10.22364/eeb.18.08 Using the Jolly-Seber model to characterise Xerolenta obvia (Gastropoda: Geomitridae) population ISSN 2255-9582 Beāte Cehanoviča1, Arturs Stalažs2* 1Dobele State Gymnasium, Dzirnavu 2, Dobele LV–3701, Latvia 2Institute of Horticulture, Graudu 1, Ceriņi, Krimūnu pagasts, Dobeles novads LV–3701, Latvia *Corresponding author, E-mail: [email protected] Abstract The terrestrial snail species Xerolenta obvia (Menke) has colonized dry, steppe-like habitats that have been created as a result of human activities in many countries outside the natural range of this species. In Latvia, this species was first recorded in 1989 in Liepāja. Observations in recent years in Liepāja have shown that snails from their initial introduction sites on the railway have also spread to the sand dune habitats within the city limits. Given that there are no snails in dune habitats that are biologically equivalent to X. obvia, this species is considered to be potentially invasive. As the distribution trends of this species in Liepāja indicate a possible threat to dry habitats in natural areas, detailed study of the species was conducted for the population of this species located in Dobele. Monitoring was performed from May 26 to August 5, 2019, carrying out 11 surveys with one week interval using the capture and re-capture method. The maximum recorded distance travelled by of one snail was 29.7 m; the calculated minimum estimated population density was 170 individuals and the maximum was 2004 individuals. Key words: alien species, Dobele population, eastern heath snail, Helicella candicans, Helicella obvia, potentially invasive species. -

Bichain Et Al.Indd
naturae 2019 ● 11 Liste de référence fonctionnelle et annotée des Mollusques continentaux (Mollusca : Gastropoda & Bivalvia) du Grand-Est (France) Jean-Michel BICHAIN, Xavier CUCHERAT, Hervé BRULÉ, Thibaut DURR, Jean GUHRING, Gérard HOMMAY, Julien RYELANDT & Kevin UMBRECHT art. 2019 (11) — Publié le 19 décembre 2019 www.revue-naturae.fr DIRECTEUR DE LA PUBLICATION : Bruno David, Président du Muséum national d’Histoire naturelle RÉDACTEUR EN CHEF / EDITOR-IN-CHIEF : Jean-Philippe Siblet ASSISTANTE DE RÉDACTION / ASSISTANT EDITOR : Sarah Figuet ([email protected]) MISE EN PAGE / PAGE LAYOUT : Sarah Figuet COMITÉ SCIENTIFIQUE / SCIENTIFIC BOARD : Luc Abbadie (UPMC, Paris) Luc Barbier (Parc naturel régional des caps et marais d’Opale, Colembert) Aurélien Besnard (CEFE, Montpellier) Vincent Boullet (Expert indépendant fl ore/végétation, Frugières-le-Pin) Hervé Brustel (École d’ingénieurs de Purpan, Toulouse) Patrick De Wever (MNHN, Paris) Thierry Dutoit (UMR CNRS IMBE, Avignon) Éric Feunteun (MNHN, Dinard) Romain Garrouste (MNHN, Paris) Grégoire Gautier (DRAAF Occitanie, Toulouse) Olivier Gilg (Réserves naturelles de France, Dijon) Frédéric Gosselin (Irstea, Nogent-sur-Vernisson) Patrick Haff ner (UMS PatriNat, Paris) Frédéric Hendoux (MNHN, Paris) Xavier Houard (OPIE, Guyancourt) Isabelle Leviol (MNHN, Concarneau) Francis Meunier (Conservatoire d’espaces naturels – Picardie, Amiens) Serge Muller (MNHN, Paris) Francis Olivereau (DREAL Centre, Orléans) Laurent Poncet (UMS PatriNat, Paris) Nicolas Poulet (AFB, Vincennes) Jean-Philippe Siblet (UMS -

Abstract Volume
ABSTRACT VOLUME August 11-16, 2019 1 2 Table of Contents Pages Acknowledgements……………………………………………………………………………………………...1 Abstracts Symposia and Contributed talks……………………….……………………………………………3-225 Poster Presentations…………………………………………………………………………………226-291 3 Venom Evolution of West African Cone Snails (Gastropoda: Conidae) Samuel Abalde*1, Manuel J. Tenorio2, Carlos M. L. Afonso3, and Rafael Zardoya1 1Museo Nacional de Ciencias Naturales (MNCN-CSIC), Departamento de Biodiversidad y Biologia Evolutiva 2Universidad de Cadiz, Departamento CMIM y Química Inorgánica – Instituto de Biomoléculas (INBIO) 3Universidade do Algarve, Centre of Marine Sciences (CCMAR) Cone snails form one of the most diverse families of marine animals, including more than 900 species classified into almost ninety different (sub)genera. Conids are well known for being active predators on worms, fishes, and even other snails. Cones are venomous gastropods, meaning that they use a sophisticated cocktail of hundreds of toxins, named conotoxins, to subdue their prey. Although this venom has been studied for decades, most of the effort has been focused on Indo-Pacific species. Thus far, Atlantic species have received little attention despite recent radiations have led to a hotspot of diversity in West Africa, with high levels of endemic species. In fact, the Atlantic Chelyconus ermineus is thought to represent an adaptation to piscivory independent from the Indo-Pacific species and is, therefore, key to understanding the basis of this diet specialization. We studied the transcriptomes of the venom gland of three individuals of C. ermineus. The venom repertoire of this species included more than 300 conotoxin precursors, which could be ascribed to 33 known and 22 new (unassigned) protein superfamilies, respectively. Most abundant superfamilies were T, W, O1, M, O2, and Z, accounting for 57% of all detected diversity.