A New Genus for the Tiny Hawk Accipiter Superciliosus and Semicollared Hawk A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Case of Cooperative Hunting by a Pair of Northern Goshawks
Acta zoologica cracoviensia, 63(2) 2020 e-ISSN 2300-0163 Kraków, 2020 https://doi.org/10.3409/azc.63.04 http://www.isez.pan.krakow.pl/en/acta-zoologica.html A case of cooperative hunting by a pair of northern goshawks Bart³omiej KUSAL and £ukasz KAJTOCH Received: 14 April 2020. Accepted: 18 September 2020. Available online: 9 October 2020. Issue online: 30 December 2020. Short communication KUSAL B., KAJTOCH £. 2020. A case of cooperative hunting by a pair of northern goshawks. Acta zool. cracov., 63(2): 21-22. Abstract. Cooperative hunting is a rare strategy in raptors, although it has been widely described in Falconidae and in some species of Accipitridae. Records about synchronous hunting in the member of the genus Accipiter are occasional. Here we describe a case of the cooperative hunting of two northern goshawks, A. gentilis, of a pigeon, Columba sp., observed in southern Poland in 2020. This exemplary behavior could be either exceptional, or cooperative hunting is an overlooked phenomenon in goshawk biology. Key words: Accipiter gentilis, Accipitridae, Columba, tandem hunting, prey. *£ukasz KAJTOCH, Bart³omiej KUSAL, Institute of Systematics and Evolution of Animals Polish Academy of Sciences, S³awkowska 17, 31-016, Kraków, Poland. E-mail: [email protected] I. INTRODUCTION and Melierax sp.). Some other birds of prey hunt in groups, but each individual preys on its own e.g. os- Cooperative hunting is a strategy that is not com- preys (Pandion haliaetus L.) and kites (Elanoides for- mon in wild animals (PACKER &RUTTAN 1988). In ficatus L., Ictinia mississippiensis (Wilson, 1811), some animal groups, cooperative hunting involves Rostrhamus sociabilis (Vieillot, 1817)). -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Chromosome Painting in Three Species of Buteoninae: a Cytogenetic Signature Reinforces the Monophyly of South American Species
Chromosome Painting in Three Species of Buteoninae: A Cytogenetic Signature Reinforces the Monophyly of South American Species Edivaldo Herculano C. de Oliveira1,2,3*, Marcella Mergulha˜o Tagliarini4, Michelly S. dos Santos5, Patricia C. M. O’Brien3, Malcolm A. Ferguson-Smith3 1 Laborato´rio de Cultura de Tecidos e Citogene´tica, SAMAM, Instituto Evandro Chagas, Ananindeua, PA, Brazil, 2 Faculdade de Cieˆncias Exatas e Naturais, ICEN, Universidade Federal do Para´, Bele´m, PA, Brazil, 3 Cambridge Resource Centre for Comparative Genomics, Cambridge, United Kingdom, 4 Programa de Po´s Graduac¸a˜oem Neurocieˆncias e Biologia Celular, ICB, Universidade Federal do Para´, Bele´m, PA, Brazil, 5 PIBIC – Universidade Federal do Para´, Bele´m, PA, Brazil Abstract Buteoninae (Falconiformes, Accipitridae) consist of the widely distributed genus Buteo, and several closely related species in a group called ‘‘sub-buteonine hawks’’, such as Buteogallus, Parabuteo, Asturina, Leucopternis and Busarellus, with unsolved phylogenetic relationships. Diploid number ranges between 2n = 66 and 2n = 68. Only one species, L. albicollis had its karyotype analyzed by molecular cytogenetics. The aim of this study was to present chromosomal analysis of three species of Buteoninae: Rupornis magnirostris, Asturina nitida and Buteogallus meridionallis using fluorescence in situ hybridization (FISH) experiments with telomeric and rDNA probes, as well as whole chromosome probes derived from Gallus gallus and Leucopternis albicollis. The three species analyzed herein showed similar karyotypes, with 2n = 68. Telomeric probes showed some interstitial telomeric sequences, which could be resulted by fusion processes occurred in the chromosomal evolution of the group, including the one found in the tassociation GGA1p/GGA6. -

An Early Pleistocene Eagle from Nebraska
248 SHORT COMMUNICATIONS kunthii blossoms by Bombus queens occurred and depend on many factors. That hummingbirds and the workers were unable to secure nectar while positioned ancestor of P. kunthii co-existed may be assumed; within the floral tube, probably as much as lo-20 otherwise its adaptation to hummingbird pollination per cent more nectar was available to Bombus p&her would make little sense. Thus it is possible that P. and Bombus trinominatus populations during this kunthii could have undergone much of its development period due to the feeding activity of Diglossa. under selective pressure from hummingbirds; still, it is clear that Diglossa baritula has co-existed with DISCUSSION AND CONCLUSIONS hummingbirds throughout New World montane hab- Grant and Grant (1968) have proposed an explanation itats for some time and therefore an earlier and more for the reciprocal evolution of hummingbirds and the important role in the evolution of P. kunthii would plants upon which they feed. According to this inter- not be unexpected. This is not to suggest that exploita- pretation most hummingbird-pollinated flowers, espe- tion late in the evolutionary development of P. kunthii cially temperate species, have evolved from bee flowers would be insignificant. Even at present, given the (Grant 1961; Grant and Grant 1965). The process potential counter-selection pressures on P. kunthii involves an incipient stage during which a primitive from bees, the presence of Dglossa perforations un- hummingbird or progenitor already “preadapted” to doubtedly precludes a certain amount of bee pollina- feed on a particular bee flower (in the sense of tion which would probably otherwise occur, helping securing insects within the corolla, or nectar, or both), to maintain the selection pressures on P. -

The Use of Green Plant Material in Bird Nests to Avoid Ectoparasites
July1984] ShortCommunications 615 the Spot-wingedFalconet may not benefitthe Monk HoY, G. 1980. Notas nidobio16gicasdel noroestear- Parakeet in such a manner. gentino. II. Physis (Buenos Aires), Secc. C, 39 We are grateful to A. G6mez Dur&n and J. C. Vera (96): 63-66. (INTA) for grantingus the useof the fieldwork areas, MACLEAN,G.L. 1973. The SociableWeaver, part 4: to N. Arguello and M. Nores for their assistancein predators, parasites and symbionts. Ostrich 44: the identification of food remains, and to J. Navarro 241-253. for his help in the field. This work wassupported by STRANECK,R., & G. VASINA. 1982. Unusual behav- a grant from the Subsecretariade Ciencia y Tecno- iour of the Spot-winged Falconet (Spiziapteryx logla (SUBCYT) of Argentina. circumcinctus).Raptor Res. 16: 25-26. LITERATURE CITED Received7 July 1983, accepted21 February1984. DEAN, A. 1971. Notes on Spiziapteryxcircumcinctus. Ibis 113: 101-102. The Use of Green Plant Material in Bird Nests to Avoid Ectoparasites PETER H. WIMBERGER 1 ZoologyDivision, Washington State Museum DB-10, Universityof Washington, Seattle,Washington 98105 USA Certain birds characteristicallyplace green plant causesnestling mortality in and nest desertion by material in their nests.This greenery is not part of birds (Webster 1944, Neff 1945, Fitch et al. 1946, Moss the nest structureproper but is placed haphazardly and Camin 1970, Feare 1976,Wheelwright and Boers- around the edges or inside the nest. The birds re- ma 1979).In general,the increasedmortality due to plenishthe spraysof greenmaterial, often daily, dur- ectoparasitesis causedby the loss of blood, which ing incubation and the nestling period (Brown and weakens the host, by viral disease, or by disease Amadon 1968, Beebe1976, pers. -
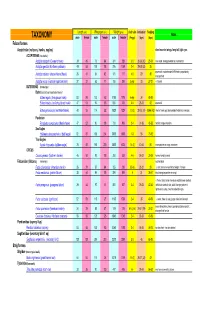
Avian Taxonomy
Length (cm) Wing span (cm) Weight (gms) cluch size incubation fledging Notes TAXONOMY male female male female male female (# eggs) (days) (days) Falconiformes Accipitridae (vultures, hawks, eagles) short rounded wings; long tail; light eyes ACCIPITRINAE (true hawks) Accipiter cooperii (Cooper's hawk) 39 45 73 84 341 528 3-5 30-36 (30) 25-34 crow sized; strongly banded tail; rounded tail Accipiter gentilis (Northern goshawk) 49 58 101 108 816 1059 2-4 28-38 (33) 35 square tail; most abundant NAM hawk; proportionaly 26 31 54 62 101 177 4-5 29 30 Accipiter striatus (sharp-shinned hawk) strongest foot Accipiter nisus (Eurasian sparrowhawk ) 37 37 62 77 150 290 5-Apr 33 27-31 Musket BUTEONINAE (broadwings) Buteo (buzzards or broad tailed hawks) Buteo regalis (ferruginous hawk) 53 59 132 143 1180 1578 6-Apr 34 45-50 Buteo lineatus (red-shouldered hawk) 47 53 96 105 550 700 3-4 28-33 42 square tail Buteo jamaicensis (red-tailed hawk) 48 55 114 122 1028 1224 1-3 (3) 28-35 (34) 42-46 (42) (Harlan' hawk spp); dark patagial featehres: immature; Parabuteo Parabuteo cuncincutus (Harris hawk) 47 52 90 108 710 890 2-4 31-36 45-50 reddish orange shoulders Sea Eagles Haliaeetus leucocephalus (bald eagle) 82 87 185 244 3000 6300 1-3 35 70-92 True Eagles Aquila chrysaetos (golden eagle) 78 82 185 220 3000 6125 1-4 (2) 40-45 50 white patches on wings: immature; CIRCUS Circus cyaneus (Northern harrier) 46 50 93 108 350 530 4-6 26-32 30-35 hovers; hunts by sound Falconidae (falcons) (longwings) notched beak Falco columbarius (American merlin) 26 29 57 64 -

Estimations Relative to Birds of Prey in Captivity in the United States of America
ESTIMATIONS RELATIVE TO BIRDS OF PREY IN CAPTIVITY IN THE UNITED STATES OF AMERICA by Roger Thacker Department of Animal Laboratories The Ohio State University Columbus, Ohio 43210 Introduction. Counts relating to birds of prey in captivity have been accomplished in some European countries; how- ever, to the knowledge of this author no such information is available in the United States of America. The following paper consistsof data related to this subject collected during 1969-1970 from surveys carried out in many different direc- tions within this country. Methods. In an attempt to obtain as clear a picture as pos- sible, counts were divided into specific areas: Research, Zoo- logical, Falconry, and Pet Holders. It became obvious as the project advanced that in some casesthere was overlap from one area to another; an example of this being a falconer working with a bird both for falconry and research purposes. In some instances such as this, the author has used his own judgment in placing birds in specific categories; in other in- stances received information has been used for this purpose. It has also become clear during this project that a count of "pets" is very difficult to obtain. Lack of interest, non-coop- eration, or no available information from animal sales firms makes the task very difficult, as unfortunately, to obtain a clear dispersal picture it is from such sourcesthat informa- tion must be gleaned. However, data related to the importa- tion of birds' of prey as recorded by the Bureau of Sport Fisheries and Wildlife is included, and it is felt some observa- tions can be made from these figures. -

SMCSP & SMCSN Wildlife List.Xlsx
Appendix C: Wildlife list for Six Mile Cypress Slough and Six Mile Cypress North Preserves Designated Status Scientific Name Common Name FWC FWS FNAI MAMMALS Family: Didelphidae (opossums) Didelphis virginiana Virginia opossum Family: Dasypodidae (armadillos) Dasypus novemcinctus nine-banded armadillo * Family: Sciuridae (squirrels and their allies) Sciurus carolinensis eastern gray squirrel Sciurus niger avicennia Big Cypress fox squirrel T G5T2/S2 Family: Muridae (mice and rats) Peromyscus gossypinus cotton mouse Oryzomys palustris marsh rice rat Sigmodon hispidus hispid cotton rat Family: Leporidae (rabbits and hares) Sylvilagus palustris marsh rabbit Sylvilagus floridanus eastern cottontail Family: Talpidae (moles) Scalopus aquaticus eastern mole Family: Felidae (cats) Puma concolor coryi Florida panther E E G5T1/S1 Lynx rufus bobcat Felis silvestris domestic cat * Family: Canidae (wolves and foxes) Urocyon cinereoargenteus common gray fox Family: Ursidae (bears) Ursus americanus floridanus Florida black bear T G5T2/S2 Family: Procyonidae (raccoons) Procyon lotor raccoon Family: Mephitidae (skunks) Spilogale putorius eastern spotted skunk Mephitis mephitis striped skunk Family: Mustelidae (weasels, otters and relatives) Lutra canadensis northern river otter Family: Suidae (old world swine) Sus scrofa feral hog * Family: Cervidae (deer) Odocoileus virginianus white-tailed deer BIRDS Family: Anatidae (swans, geese and ducks) Subfamily: Anatinae (dabbling ducks) Dendrocygna autumnalis black-bellied whistling duck Cairina moschata muscovy -

Repeated Sequence Homogenization Between the Control and Pseudo
Repeated Sequence Homogenization Between the Control and Pseudo-Control Regions in the Mitochondrial Genomes of the Subfamily Aquilinae LUIS CADAH´IA1*, WILHELM PINSKER1, JUAN JOSE´ NEGRO2, 3 4 1 MIHAELA PAVLICEV , VICENTE URIOS , AND ELISABETH HARING 1Molecular Systematics, 1st Zoological Department, Museum of Natural History Vienna, Vienna, Austria 2Department of Evolutionary Ecology, Estacio´n Biolo´gica de Don˜ana, Sevilla, Spain 3Centre of Ecological and Evolutionary Synthesis (CEES), Department of Biology, Faculty for Natural Sciences and Math, University of Oslo, Oslo, Norway 4Estacio´n Biolo´gica Terra Natura (Fundacio´n Terra Natura—CIBIO), Universidad de Alicante, Alicante, Spain ABSTRACT In birds, the noncoding control region (CR) and its flanking genes are the only parts of the mitochondrial (mt) genome that have been modified by intragenomic rearrangements. In raptors, two noncoding regions are present: the CR has shifted to a new position with respect to the ‘‘ancestral avian gene order,’’ whereas the pseudo-control region (CCR) is located at the original genomic position of the CR. As possible mechanisms for this rearrangement, duplication and transposition have been considered. During characterization of the mt gene order in Bonelli’s eagle Hieraaetus fasciatus, we detected intragenomic sequence similarity between the two regions supporting the duplication hypothesis. We performed intra- and intergenomic sequence comparisons in H. fasciatus and other falconiform species to trace the evolution of the noncoding mtDNA regions in Falconiformes. We identified sections displaying different levels of similarity between the CR and CCR. On the basis of phylogenetic analyses, we outline an evolutionary scenario of the underlying mutation events involving duplication and homogenization processes followed by sporadic deletions. -

Molecular Phylogenetics of the Buteonine Birds of Prey (Accipitridae)
'e Auk 304(2):304–315, 2008 )e American Ornithologists’ Union, 2008. Printed in USA. MOLECULAR PHYLOGENETICS OF THE BUTEONINE BIRDS OF PREY (ACCIPITRIDAE) HEATHER R. L. LERNER,1 MATTHEW C. KLAVER, AND DAVID P. MINDELL2 Museum of Zoology and Department of Ecology and Evolutionary Biology, University of Michigan, 1109 Geddes Avenue, Ann Arbor, Michigan 48109, USA A.—Phylogenetic relationships among birds of prey in thhee subbffamily Buteoninae are not fully established but are of par- ticular interest because the Buteoninae constitute one of the largest accipitrid subgroups and include multiple species of conservation concern. Genera previously included within the Buteoninae are Buteo, Leucopternis, Buteogallus, Harpyhaliaetus, Busarellus, Parabu- teo, Geranoaetus, Geranospiza, Ictinia, Rostrhamus, Kaupifalco, and Butastur. We analyzed representatives from all buteonine genera and most non-Buteo (i.e., “sub-buteo”) species with , bases of nuclear and mitochondrial DNA and found non-monophyly for the nominal genera Buteo, Buteogallus, and Leucopternis. )e Old World Lizard Buzzard (Kaupifalco monogrammicus) is not closely re- lated to buteonine taxa but is sister to goshawks in the genera Melierax, Micronisus, and Urotriorchis. Another Old World genus, Butas- tur, is sister to the clade including all other buteonine genera mentioned above. Investigation of several “superspecies” complexes within the genus Leucopternis revealed non-monophyly for the four subspecies of White Hawk (L. albicollis). On the basis of mitochondrial data, L. a. albicollis forms a clade with L. polionotus, whereas L. a. costaricensis, L. a. ghiesbreghti, and L. a. williaminae form a clade with L. occidentalis. Among taxa included as outgroups, we found two species in the genus Circus to be clearly nested within a clade of Accipiter spp. -

Common Birds of Namibia and Botswana 1 Josh Engel
Common Birds of Namibia and Botswana 1 Josh Engel Photos: Josh Engel, [[email protected]] Integrative Research Center, Field Museum of Natural History and Tropical Birding Tours [www.tropicalbirding.com] Produced by: Tyana Wachter, R. Foster and J. Philipp, with the support of Connie Keller and the Mellon Foundation. © Science and Education, The Field Museum, Chicago, IL 60605 USA. [[email protected]] [fieldguides.fieldmuseum.org/guides] Rapid Color Guide #584 version 1 01/2015 1 Struthio camelus 2 Pelecanus onocrotalus 3 Phalacocorax capensis 4 Microcarbo coronatus STRUTHIONIDAE PELECANIDAE PHALACROCORACIDAE PHALACROCORACIDAE Ostrich Great white pelican Cape cormorant Crowned cormorant 5 Anhinga rufa 6 Ardea cinerea 7 Ardea goliath 8 Ardea pupurea ANIHINGIDAE ARDEIDAE ARDEIDAE ARDEIDAE African darter Grey heron Goliath heron Purple heron 9 Butorides striata 10 Scopus umbretta 11 Mycteria ibis 12 Leptoptilos crumentiferus ARDEIDAE SCOPIDAE CICONIIDAE CICONIIDAE Striated heron Hamerkop (nest) Yellow-billed stork Marabou stork 13 Bostrychia hagedash 14 Phoenicopterus roseus & P. minor 15 Phoenicopterus minor 16 Aviceda cuculoides THRESKIORNITHIDAE PHOENICOPTERIDAE PHOENICOPTERIDAE ACCIPITRIDAE Hadada ibis Greater and Lesser Flamingos Lesser Flamingo African cuckoo hawk Common Birds of Namibia and Botswana 2 Josh Engel Photos: Josh Engel, [[email protected]] Integrative Research Center, Field Museum of Natural History and Tropical Birding Tours [www.tropicalbirding.com] Produced by: Tyana Wachter, R. Foster and J. Philipp, -

Quantifying the Global Legal Trade in Live CITES-Listed Raptors and Owls
Electronic Supplementary Material (Panter et al. 2019) Electronic Supplementary Material for: Quantifying the global legal trade in live CITES-listed raptors and owls for commercial purposes over a 40-year period Published in 2019 in Avocetta 43(1) :23-36; doi: https://doi.org/10.30456/AVO.2019104 Authors: Connor T. Panter1,*, Eleanor D. Atkinson1, Rachel L. White1 1 School of Pharmacy and Biomolecular Sciences, University of Brighton, Brighton, United Kingdom. * Corresponding author: [email protected] List of contents: ESM 1 - Appendix A. CITES source categories with associated definitions. ESM 2 - Appendix B. CITES Trade Purposes categories with associated definitions. ESM 3 - Appendix C. CITES Importer and Exporter countries with total reported imported and exported individuals of raptors and owls. ESM 4 - Appendix D. Raptor and owl exporter countries supplying the Japanese trade in live birds for commercial use. ESM 5 - Appendix E. Percentages of number of traded species within global IUCN Red List categories and population trends. ESM 6. Imported raptor species, number of imported individuals and percentage of total imported raptor individuals. ESM 7. Exported raptor species, number of exported individuals and percentage of total exported raptor individuals. ESM 8. Imported owl species, number of imported individuals and percentage of total imported owl individuals. ESM 9. Exported owl species, number of exported individuals and percentage of total exported owl individuals. 1 Electronic Supplementary Material (Panter et al. 2019) ELECTRONIC SUPPLEMENTARY MATERIAL (ESM) ESM 1 - Appendix A. CITES source categories with associated definitions. *The CITES Trade Database does not provide information regarding whether birds declared as “wild- caught” were derived from legal or illegal activities.