Repeated Sequence Homogenization Between the Control and Pseudo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Multi-Gene Phylogeny of Aquiline Eagles (Aves: Accipitriformes) Reveals Extensive Paraphyly at the Genus Level
Available online at www.sciencedirect.com MOLECULAR SCIENCE•NCE /W\/Q^DIRI DIRECT® PHYLOGENETICS AND EVOLUTION ELSEVIER Molecular Phylogenetics and Evolution 35 (2005) 147-164 www.elsevier.com/locate/ympev A multi-gene phylogeny of aquiline eagles (Aves: Accipitriformes) reveals extensive paraphyly at the genus level Andreas J. Helbig'^*, Annett Kocum'^, Ingrid Seibold^, Michael J. Braun^ '^ Institute of Zoology, University of Greifswald, Vogelwarte Hiddensee, D-18565 Kloster, Germany Department of Zoology, National Museum of Natural History, Smithsonian Institution, 4210 Silver Hill Rd., Suitland, MD 20746, USA Received 19 March 2004; revised 21 September 2004 Available online 24 December 2004 Abstract The phylogeny of the tribe Aquilini (eagles with fully feathered tarsi) was investigated using 4.2 kb of DNA sequence of one mito- chondrial (cyt b) and three nuclear loci (RAG-1 coding region, LDH intron 3, and adenylate-kinase intron 5). Phylogenetic signal was highly congruent and complementary between mtDNA and nuclear genes. In addition to single-nucleotide variation, shared deletions in nuclear introns supported one basal and two peripheral clades within the Aquilini. Monophyly of the Aquilini relative to other birds of prey was confirmed. However, all polytypic genera within the tribe, Spizaetus, Aquila, Hieraaetus, turned out to be non-monophyletic. Old World Spizaetus and Stephanoaetus together appear to be the sister group of the rest of the Aquilini. Spiza- stur melanoleucus and Oroaetus isidori axe nested among the New World Spizaetus species and should be merged with that genus. The Old World 'Spizaetus' species should be assigned to the genus Nisaetus (Hodgson, 1836). The sister species of the two spotted eagles (Aquila clanga and Aquila pomarina) is the African Long-crested Eagle (Lophaetus occipitalis). -

Chromosome Painting in Three Species of Buteoninae: a Cytogenetic Signature Reinforces the Monophyly of South American Species
Chromosome Painting in Three Species of Buteoninae: A Cytogenetic Signature Reinforces the Monophyly of South American Species Edivaldo Herculano C. de Oliveira1,2,3*, Marcella Mergulha˜o Tagliarini4, Michelly S. dos Santos5, Patricia C. M. O’Brien3, Malcolm A. Ferguson-Smith3 1 Laborato´rio de Cultura de Tecidos e Citogene´tica, SAMAM, Instituto Evandro Chagas, Ananindeua, PA, Brazil, 2 Faculdade de Cieˆncias Exatas e Naturais, ICEN, Universidade Federal do Para´, Bele´m, PA, Brazil, 3 Cambridge Resource Centre for Comparative Genomics, Cambridge, United Kingdom, 4 Programa de Po´s Graduac¸a˜oem Neurocieˆncias e Biologia Celular, ICB, Universidade Federal do Para´, Bele´m, PA, Brazil, 5 PIBIC – Universidade Federal do Para´, Bele´m, PA, Brazil Abstract Buteoninae (Falconiformes, Accipitridae) consist of the widely distributed genus Buteo, and several closely related species in a group called ‘‘sub-buteonine hawks’’, such as Buteogallus, Parabuteo, Asturina, Leucopternis and Busarellus, with unsolved phylogenetic relationships. Diploid number ranges between 2n = 66 and 2n = 68. Only one species, L. albicollis had its karyotype analyzed by molecular cytogenetics. The aim of this study was to present chromosomal analysis of three species of Buteoninae: Rupornis magnirostris, Asturina nitida and Buteogallus meridionallis using fluorescence in situ hybridization (FISH) experiments with telomeric and rDNA probes, as well as whole chromosome probes derived from Gallus gallus and Leucopternis albicollis. The three species analyzed herein showed similar karyotypes, with 2n = 68. Telomeric probes showed some interstitial telomeric sequences, which could be resulted by fusion processes occurred in the chromosomal evolution of the group, including the one found in the tassociation GGA1p/GGA6. -

Status of the Eastern Imperial Eagle (Aquila Heliaca) in the European Part of Turkey
ACTA ZOOLOGICA BULGARICA Acta zool. bulg., Suppl. 3, 2011: 87-93 Status of the Eastern Imperial Eagle (Aquila heliaca) in the European part of Turkey Dimitar A. Demerdzhiev1, Stoycho A. Stoychev2, Nikolay G. Terziev2, and Ivaylo D. Angelov2 1 31 Bulgaria Blv�., 4230 Asenovgra�, Bulgaria; E�mails: �emer�jiev@yahoo.�om; �_�emer�[email protected]; w��.bspb.org 2 Haskovo 6300, P.O.Box 130, Bulgaria; E�mails: stoy�hev.s@gmail.�om; w��.bspb.org; [email protected]; ivailoange� [email protected]; w��.bspb.org Abstract: This arti�le presents the results of the �rst more �etaile� stu�ying on the �istribution an� numbers of the Eastern Imperial Eagle (Аquila heliaca SA V I G NY 1809) population in the European part of Turkey. T�enty territories o��upie� by Imperial Eagle pairs, �istribute� in three �ifferent regions �ere �is�overe� �uring the perio� 2008�2009. The bree�ing population was estimate� at 30�50 pairs. The stu�y i�enti�e� t�o main habitat types typi�al of the Imperial Eagles in European Turkey – open hilly areas an� lo� mountain areas (up to 450 m a.s.l.) an� lo� relief plain areas (50�150 m a.s.l.). Poplar trees (Populus sp. L) were i�enti�e� as the most preferre� nesting substratum (44%), follo�e� by Oaks (Quercus sp. L) (40%). Bree�ing �ensity �as 1 pair/100 km2 in both habitat types. The shortest �istan�e bet�een t�o bree�ing pairs �as 5.8 km re�or�e� in plain areas in the Thra�e region. -

An Early Pleistocene Eagle from Nebraska
248 SHORT COMMUNICATIONS kunthii blossoms by Bombus queens occurred and depend on many factors. That hummingbirds and the workers were unable to secure nectar while positioned ancestor of P. kunthii co-existed may be assumed; within the floral tube, probably as much as lo-20 otherwise its adaptation to hummingbird pollination per cent more nectar was available to Bombus p&her would make little sense. Thus it is possible that P. and Bombus trinominatus populations during this kunthii could have undergone much of its development period due to the feeding activity of Diglossa. under selective pressure from hummingbirds; still, it is clear that Diglossa baritula has co-existed with DISCUSSION AND CONCLUSIONS hummingbirds throughout New World montane hab- Grant and Grant (1968) have proposed an explanation itats for some time and therefore an earlier and more for the reciprocal evolution of hummingbirds and the important role in the evolution of P. kunthii would plants upon which they feed. According to this inter- not be unexpected. This is not to suggest that exploita- pretation most hummingbird-pollinated flowers, espe- tion late in the evolutionary development of P. kunthii cially temperate species, have evolved from bee flowers would be insignificant. Even at present, given the (Grant 1961; Grant and Grant 1965). The process potential counter-selection pressures on P. kunthii involves an incipient stage during which a primitive from bees, the presence of Dglossa perforations un- hummingbird or progenitor already “preadapted” to doubtedly precludes a certain amount of bee pollina- feed on a particular bee flower (in the sense of tion which would probably otherwise occur, helping securing insects within the corolla, or nectar, or both), to maintain the selection pressures on P. -
Avian Taxonomy
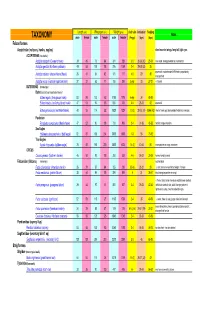
Length (cm) Wing span (cm) Weight (gms) cluch size incubation fledging Notes TAXONOMY male female male female male female (# eggs) (days) (days) Falconiformes Accipitridae (vultures, hawks, eagles) short rounded wings; long tail; light eyes ACCIPITRINAE (true hawks) Accipiter cooperii (Cooper's hawk) 39 45 73 84 341 528 3-5 30-36 (30) 25-34 crow sized; strongly banded tail; rounded tail Accipiter gentilis (Northern goshawk) 49 58 101 108 816 1059 2-4 28-38 (33) 35 square tail; most abundant NAM hawk; proportionaly 26 31 54 62 101 177 4-5 29 30 Accipiter striatus (sharp-shinned hawk) strongest foot Accipiter nisus (Eurasian sparrowhawk ) 37 37 62 77 150 290 5-Apr 33 27-31 Musket BUTEONINAE (broadwings) Buteo (buzzards or broad tailed hawks) Buteo regalis (ferruginous hawk) 53 59 132 143 1180 1578 6-Apr 34 45-50 Buteo lineatus (red-shouldered hawk) 47 53 96 105 550 700 3-4 28-33 42 square tail Buteo jamaicensis (red-tailed hawk) 48 55 114 122 1028 1224 1-3 (3) 28-35 (34) 42-46 (42) (Harlan' hawk spp); dark patagial featehres: immature; Parabuteo Parabuteo cuncincutus (Harris hawk) 47 52 90 108 710 890 2-4 31-36 45-50 reddish orange shoulders Sea Eagles Haliaeetus leucocephalus (bald eagle) 82 87 185 244 3000 6300 1-3 35 70-92 True Eagles Aquila chrysaetos (golden eagle) 78 82 185 220 3000 6125 1-4 (2) 40-45 50 white patches on wings: immature; CIRCUS Circus cyaneus (Northern harrier) 46 50 93 108 350 530 4-6 26-32 30-35 hovers; hunts by sound Falconidae (falcons) (longwings) notched beak Falco columbarius (American merlin) 26 29 57 64 -

Separation of Tawny Eagle from Steppe Eagle In
Notes Separation of Tawny Eagle from Steppe Eagle in Israel Tawny Eagle Aquik rapax generally looks smaller and rather puny (and also, when perched, less elongated/horizontal) compared with Steppe Eagle A. nipaknsis. Diagnostically, the North African race A. r. belisarius differs in all plumages from Steppe in being smaller, in having die creamy-white of the uppertail- coverts extending well up onto the upper back (on Steppe, usually restricted to uppertail-coverts, lacking on rump and only indistinct on lower back), in showing a conspicuous sandy wedge on the inner primaries below (generally indistinct or absent on Steppe) and, in juvenile/immature plumages, lacking Steppe's whitish underwing-band; the remiges and rectrices are usually finely barred or almost unbarred compared widi corresponding plumages of Steppe. The general coloration of Tawny's body and underwing-coverts is pale tawny or bufEsh-yellow to pale foxy/rufous (or dark greyish-brown, but this is virtu ally confined to more soumerly populations/races), contrasting highly widi the remiges and rectrices: more or less reminiscent of the underpart pattern of juvenile Imperial Eagle A. heliaca, but unstreaked. The mantle, scapulars and upperwing-coverts are also distincdy tawny-sandy, but with the feathers dark- centred (chiefly on greater coverts, tertials and lower scapulars). In good views, mainly when perched, Tawny normally shows a slighdy shorter gape line, ending level widi the centre of die eye (reaches rear edge on Steppe); die adult's iris is yellowish-brown (dark brown on Steppe). In flight, compared wim Steppe, Tawny has broad and short wings with ample 'hand', and shows a well-protruding head and a relatively short and more square-ended tail; in active flight, it is rather stiff-winged with more rapid wingbeats. -

Mimicry in Birds of Prey Published 4 April 2013
Brazilian raptors (Online publications) www.avesderapinabrasil.com Mimicry in birds of prey Published 4 April 2013 Juvenile Grey-headed Kite (Leptodon cayanensis) with a plumage pattern that imitates almost perfectly an Ornate Hawk-Eagle (Spizaetus ornatus). Belterra (Pará), March 2013. Photo: Eleonora Pinheiro Willian Menq 1 Email: [email protected] Translated by Johan Ingels. Mimicry in birds of prey is impressive although uncommon. Some predators use mimicry as a hunting strategy. This is the case with the Zone-tailed Hawk (Buteo albonotatus) which imitates the inoffensive vultures of the genus Cathartes to approach prey (Peckhamian mimicry). But a mimic that mimics another more dangerous species, seems to be the most common type of mimicry among hawks in Brazil. So, the Rufous-thighed Kite (Harpagus diodon) which has a predominantly insectivorous diet mimics a Bicoloured Hawk (Accipiter bicolor) which mostly hunts birds. Other species of raptors only utilise mimicry as a juvenile, which is the case with the Grey- bellied Hawk (Accipiter poliogaster) and the Grey-headed Kite (Leptodon cayanensis). The juvenile plumage of the Grey-bellied Hawk is very similar to the adult plumage of the Ornate Hawk-Eagle (Spizaetus ornatus), possibly as a defense against other hawks and large primates that could prey on its nest. The juvenile Grey-headed Kites are highly polymorphic, presenting diverse plumage patterns. There are totally white individuals that mimic the Black-and-white Hawk-Eagle (Spizaetus melanoleucus), others are almost completely black resembling a Black Hawk-Eagle (Spizaetus tyrannus), and there are some rare intermediate forms that have little 1 MENQ, W. (2013). -

SMCSP & SMCSN Wildlife List.Xlsx
Appendix C: Wildlife list for Six Mile Cypress Slough and Six Mile Cypress North Preserves Designated Status Scientific Name Common Name FWC FWS FNAI MAMMALS Family: Didelphidae (opossums) Didelphis virginiana Virginia opossum Family: Dasypodidae (armadillos) Dasypus novemcinctus nine-banded armadillo * Family: Sciuridae (squirrels and their allies) Sciurus carolinensis eastern gray squirrel Sciurus niger avicennia Big Cypress fox squirrel T G5T2/S2 Family: Muridae (mice and rats) Peromyscus gossypinus cotton mouse Oryzomys palustris marsh rice rat Sigmodon hispidus hispid cotton rat Family: Leporidae (rabbits and hares) Sylvilagus palustris marsh rabbit Sylvilagus floridanus eastern cottontail Family: Talpidae (moles) Scalopus aquaticus eastern mole Family: Felidae (cats) Puma concolor coryi Florida panther E E G5T1/S1 Lynx rufus bobcat Felis silvestris domestic cat * Family: Canidae (wolves and foxes) Urocyon cinereoargenteus common gray fox Family: Ursidae (bears) Ursus americanus floridanus Florida black bear T G5T2/S2 Family: Procyonidae (raccoons) Procyon lotor raccoon Family: Mephitidae (skunks) Spilogale putorius eastern spotted skunk Mephitis mephitis striped skunk Family: Mustelidae (weasels, otters and relatives) Lutra canadensis northern river otter Family: Suidae (old world swine) Sus scrofa feral hog * Family: Cervidae (deer) Odocoileus virginianus white-tailed deer BIRDS Family: Anatidae (swans, geese and ducks) Subfamily: Anatinae (dabbling ducks) Dendrocygna autumnalis black-bellied whistling duck Cairina moschata muscovy -

Nesting Pair Density and Abundance of Ferruginous Hawks (Buteo Regalis) and Golden Eagles (Aquila Chrysaetos) from Aerial Surveys in Wyoming
J. Raptor Res. 49(4):400–412 E 2015 The Raptor Research Foundation, Inc. NESTING PAIR DENSITY AND ABUNDANCE OF FERRUGINOUS HAWKS (BUTEO REGALIS) AND GOLDEN EAGLES (AQUILA CHRYSAETOS) FROM AERIAL SURVEYS IN WYOMING LUCRETIA E. OLSON1 U.S.D.A., Rocky Mountain Research Station, Missoula, MT 59801 U.S.A. ROBERT J. OAKLEAF Wyoming Department of Game and Fish, Lander, WY 82520 U.S.A. JOHN R. SQUIRES U.S.D.A., Rocky Mountain Research Station, Missoula, MT 59801 U.S.A. ZACHARY P. WALLACE AND PATRICIA L. KENNEDY Eastern Oregon Agriculture and Natural Resource Program and Department of Fisheries and Wildlife, Oregon State University, Union, OR 97331 U.S.A. ABSTRACT.—Raptors that inhabit sagebrush steppe and grassland ecosystems in the western United States may be threatened by continued loss and modification of their habitat due to energy development, con- version to agriculture, and human encroachment. Actions to protect these species are hampered by a lack of reliable data on such basic information as population size and density. We estimated density and abundance of nesting pairs of Ferruginous Hawks (Buteo regalis) and Golden Eagles (Aquila chrysaetos)in sagebrush steppe and grassland regions of Wyoming, based on aerial line transect surveys of randomly selected townships. In 2010 and 2011, we surveyed 99 townships and located 62 occupied Ferruginous Hawk nests and 36 occupied Golden Eagle nests. We used distance sampling to estimate a nesting pair density of 94.7 km2 per pair (95% CI: 69.9–139.8 km2) for Ferruginous Hawks, and 165.9 km2 per pair (95% CI: 126.8–230.8 km2) for Golden Eagles. -

Molecular Phylogenetics of the Buteonine Birds of Prey (Accipitridae)
'e Auk 304(2):304–315, 2008 )e American Ornithologists’ Union, 2008. Printed in USA. MOLECULAR PHYLOGENETICS OF THE BUTEONINE BIRDS OF PREY (ACCIPITRIDAE) HEATHER R. L. LERNER,1 MATTHEW C. KLAVER, AND DAVID P. MINDELL2 Museum of Zoology and Department of Ecology and Evolutionary Biology, University of Michigan, 1109 Geddes Avenue, Ann Arbor, Michigan 48109, USA A.—Phylogenetic relationships among birds of prey in thhee subbffamily Buteoninae are not fully established but are of par- ticular interest because the Buteoninae constitute one of the largest accipitrid subgroups and include multiple species of conservation concern. Genera previously included within the Buteoninae are Buteo, Leucopternis, Buteogallus, Harpyhaliaetus, Busarellus, Parabu- teo, Geranoaetus, Geranospiza, Ictinia, Rostrhamus, Kaupifalco, and Butastur. We analyzed representatives from all buteonine genera and most non-Buteo (i.e., “sub-buteo”) species with , bases of nuclear and mitochondrial DNA and found non-monophyly for the nominal genera Buteo, Buteogallus, and Leucopternis. )e Old World Lizard Buzzard (Kaupifalco monogrammicus) is not closely re- lated to buteonine taxa but is sister to goshawks in the genera Melierax, Micronisus, and Urotriorchis. Another Old World genus, Butas- tur, is sister to the clade including all other buteonine genera mentioned above. Investigation of several “superspecies” complexes within the genus Leucopternis revealed non-monophyly for the four subspecies of White Hawk (L. albicollis). On the basis of mitochondrial data, L. a. albicollis forms a clade with L. polionotus, whereas L. a. costaricensis, L. a. ghiesbreghti, and L. a. williaminae form a clade with L. occidentalis. Among taxa included as outgroups, we found two species in the genus Circus to be clearly nested within a clade of Accipiter spp. -

Winter Ranging Behaviour of a Greater Spotted Eagle (Aquila
Slovak Raptor Journal 2014, 8(2): 123–128. DOI: 10.2478/srj-2014-0014. © Raptor Protection ofSlovakia (RPS) Winter ranging behaviour of a greater spotted eagle (Aquila clanga) in south- east Spain during four consecutive years Zimné teritoriálne správanie orla hrubozobého (Aquila clanga) v juhovýchodnom Španiel- sku počas štyroch za sebou nasledujúcich rokov Juan M. PÉREZ-GARCÍA, Urmas SELLIS & Ülo VÄLI Abstract: Knowing the winter behaviour is essential for deciding on conservation strategies for threatened migratory species such as the greater spotted eagle (Aquila clanga). Fidelity and inter-annual variation in winter home range of an Estonian greater spotted eagle were studied during the first four years of its life by means of GPS satellite telemetry in south-eastern Spain. Results show the eagle exploited a small area (12.7 km2, 95% kernel) with high inter-annual fidelity during all winter stages. The A. clanga preferred marshes and water bodies and avoided irrigated crops and urban areas. Waterfowl hunting did not show any effect on the spatial pattern of the eagle’s behaviour, although water level management in reservoirs could influence their use by the A. clanga. Our study highlights that the wintering home range may be limited to a small suitable habitat patch where human activities, especially water reservoir management, should be regulated. Abstrakt: Nevyhnutným predpokladom efektívnych ochranárskych opatrení je poznanie zimného správania ohrozeného mi- grujúceho druhu, akým je orol hrubozobý (Aquila clanga). Fidelita a medziročná variabilita charakteristík zimného okrsku jedinca orla hrubozobého pochádzajúceho z Estónska sa sledovala počas prvých štyroch rokov jeho života pomocou GPS-satelit- nej telemetrie v juhovýchodnom Španielsku. -

Part Ii. Zoölogy
Text extracted from a scan by Google Book Search. satisfactory account of the exact progress of the work, or even to embody the results accomplished when so much FIRST BIENNIAL REPORT remains unfinished. OF THE The subjoined catalogue of the species known to inhabit PROGRESS our State, will, perhaps, best present an outline of the OF THE labor already performed, and at the same time furnish GEOLOGICAL SURVEY desirable information in regard to the geographical range OF MICHIGAN, of species. EMBRACING OBSERVATIONS ON THE In addition to the list here presented there are large GEOLOGY, ZOÖLOGY, AND BOTANY numbers of specimens that remain to be identified and OF THE described, which will materially increase the number of LOWER PENINSULA known species in the State. The fishes, insects, and crustaceans have not been worked up and for that reason have been omitted from MADE TO THE GOVERNOR, DECEMBER 31, 1860. the catalogue. BY AUTHORITY. It may not be out of place in this connection to make a brief statement of the aims to be kept in view, and the LANSING: results which may be expected to follow from the earnest Hosmer & Kerr, Printers to the State. prosecution of the study of the Zoology of our State. 1861. From the intimate and important relations existing Digitized by Google between man and the various branches of the Animal REPORT OF THE STATE GEOLOGIST. kingdom, he is particularly interested in becoming acquainted with the forms, structure, metamorphoses, habits, and dispositions of the animate beings which surround him. He would thus be better fitted to act intelligently in availing himself of the benefits to be PART II.