ᅡᆳgeometric Morphometric Analysis of Snout Shape in Extant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

O18934 Plard, F., Bonenfant, C. and Gaillard, JM 2011
Oikos O18934 Plard, F., Bonenfant, C. and Gaillard, J. M. 2011. Re- visiting the allometry of antlers among deer species: male-male sexual competition as a driver. – Oikos 120: 601–606. Table A1. Data basis. Sexual size dimorphism (SSD) was measured by the residuals of the regression of male body mass against female body mass. Breeding group sizes (BGS) were divided into three categories: group of one or two animals (A), group of three, four and five animals (B), and groups larger than five animals (C). The different mating tactics (MT) were harem (H), territorial (T) and tending (F). Non-available mating tactics (NA) and monogamous species (M) are indicated. Sub-family Genus Species Common name Male Female SSD BGS MT Ref Antler Body Body length mass mass Cervinae Axis axis chital 845 89.5 39 0.58 C F 1,2,3 Cervinae Axis porcinus hog deer 399 41 31 0.07 C F 2,3,4,5 Cervinae Cervus albirostris white-lipped deer 1150 204 125 0.06 C H 1,6 Cervinae Cervus canadensis wapiti 1337 350 250 –0.21 C H 3,8,9 Cervinae Cervus duvaucelii barasingha 813 236 145 0.03 C H 1,3,10,11 Cervinae Cervus elaphus red deer 936 250 125 0.34 C H 1,2,3 Cervinae Cervus eldi Eld’s deer 971.5 105 67 0.12 C H 1,2,3 Cervinae Cervus nippon sika deer 480 52 37 0.1 B T 1,2,3,9,12,13 Cervinae Cervus timorensis Timor deer 675 95.5 53 0.29 C H 1,9 Cervinae Cervus unicolor sambar 1049 192 146 –0.18 B T 1,2,3,7 Cervinae Dama dama fallow deer 615 67 44 0.15 C T 1,2,3,14 Cervinae Elaphurus davidianus Père David’s deer 737 214 159 –0.17 C H 1,2,3 Muntiacinae Elaphodus cephalophus -

Friday Night Auction
GETTING YOUTH OUTDOORS Welcome Dear Friends and Fellow Outdoor Supporters, Welcome and thank you for supporting the second annual Heartland DSC Banquet! Heartland DSC is excited to state that during the last year we were able to successfully pursue our mission of “Getting Youth Outdoors” by exposing kids to hunting and fishing that may have not otherwise had the opportunity. Highlights of the past year included a youth fishing tournament, certification of new young hunters during our hunter education course, a youth shooting clinic and Heartland DSC sponsored youth hunts. For many of the young participants it was their first time doing these types of activities. We took a large step in providing hunting opportunities for young people by securing exclusive access to a tract of land teaming with deer and turkey. This provides us the ability to offer youth hunting in a well-controlled environment. While our efforts are focused on all youth, we are putting a special emphasis on introducing outdoor activities to those youth that may not otherwise have the opportunity due to disabilities, lack of accessibility, or other life circumstances. We have worked hard to attract outstanding auction items from reputable outfitters and donors who have graciously donated to the organization’s mission. Heartland DSC encourages you to place your bids with confidence and in the spirit of providing the funds necessary to further the organization’s objectives while obtaining goods and services for your enjoyment. Again, thank you for your support and have a wonderful evening! Corey Goss & Family Heartland DSC President GETTING YOUTH OUTDOORS Schedule of Events Saturday March 2, 2019 NOON: Lunch with Larry Weishuhn Pasta and Nacho Bars 5:00 PM: Social Time / Cash Bar Silent Auction Opens Raffles Games 7:00 PM: Special Guest Speaker Larry Weishuhn Dinner 7:30 PM: Silent Auction Closes 8:00 PM: Live Auction Begins Heartland DSC About Heartland DSC Heartland DSC is the local chapter of Dallas Safari Club (DSC) with members throughout Iowa and Nebraska. -

Fitzhenry Yields 2016.Pdf
Stellenbosch University https://scholar.sun.ac.za ii DECLARATION By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. Date: March 2016 Copyright © 2016 Stellenbosch University All rights reserved Stellenbosch University https://scholar.sun.ac.za iii GENERAL ABSTRACT Fallow deer (Dama dama), although not native to South Africa, are abundant in the country and could contribute to domestic food security and economic stability. Nonetheless, this wild ungulate remains overlooked as a protein source and no information exists on their production potential and meat quality in South Africa. The aim of this study was thus to determine the carcass characteristics, meat- and offal-yields, and the physical- and chemical-meat quality attributes of wild fallow deer harvested in South Africa. Gender was considered as a main effect when determining carcass characteristics and yields, while both gender and muscle were considered as main effects in the determination of physical and chemical meat quality attributes. Live weights, warm carcass weights and cold carcass weights were higher (p < 0.05) in male fallow deer (47.4 kg, 29.6 kg, 29.2 kg, respectively) compared with females (41.9 kg, 25.2 kg, 24.7 kg, respectively), as well as in pregnant females (47.5 kg, 28.7 kg, 28.2 kg, respectively) compared with non- pregnant females (32.5 kg, 19.7 kg, 19.3 kg, respectively). -

Gaits and Their Development in the Infraorder Pecora by Anne Innis
Gaits and Their Development in the Infraorder Pecora by Anne Innis Dagg, M.A. THESIS Presented to the Faculty of Graduate Studies of the University of Waterloo in partial ful fillmenc of the requirements for the degree of Doctor of Philosopty in Biolog The University of Waterloo February, 1967 The University of Waterloo requires the signature of all persons using this thesis. Please sign below, and give address and date. I hereby declare that I am the sole a�thor of this thesis. I authorize the University of Waterloo to lend it to other institutions or individuals for the purpose of scholarly research. Signature Abstract The gaits of twenty-eight species of the Infraorder Pecora are analyzed from motion picture sequences comprising over 45,000 frames. For each gait the percentage time spent on the various supporting legs during each stride and the order in which the combinations of supporting legs were used are tabulated. When possible the times for the strides are calculated. The walk patterns of each of the four families studied are shown to be statistically distinctive. Within each family, those members that live where the vegetation is so dense that they must rely on hearing to warn them of danger use a more stable walk than those species that inhabit open grasslands. A stable walk is that in which diagonal legs are used in·preference to lateral legs and three or four supporting legs are used rather than two. With a more stable walk, 4 species is able to pause quickly and to flee instantly if danger thr�atens. -
Summary of Protected Areas in Chad
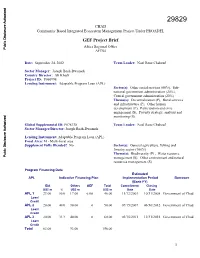
CHAD Community Based Integrated Ecosystem Management Project Under PROADEL GEF Project Brief Africa Regional Office Public Disclosure Authorized AFTS4 Date: September 24, 2002 Team Leader: Noel Rene Chabeuf Sector Manager: Joseph Baah-Dwomoh Country Director: Ali Khadr Project ID: P066998 Lending Instrument: Adaptable Program Loan (APL) Sector(s): Other social services (60%), Sub- national government administration (20%), Central government administration (20%) Theme(s): Decentralization (P), Rural services Public Disclosure Authorized and infrastructure (P), Other human development (P), Participation and civic engagement (S), Poverty strategy, analysis and monitoring (S) Global Supplemental ID: P078138 Team Leader: Noel Rene Chabeuf Sector Manager/Director: Joseph Baah-Dwomoh Lending Instrument: Adaptable Program Loan (APL) Focal Area: M - Multi-focal area Supplement Fully Blended? No Sector(s): General agriculture, fishing and forestry sector (100%) Theme(s): Biodiversity (P) , Water resource Public Disclosure Authorized management (S), Other environment and natural resources management (S) Program Financing Data Estimated APL Indicative Financing Plan Implementation Period Borrower (Bank FY) IDA Others GEF Total Commitment Closing US$ m % US$ m US$ m Date Date APL 1 23.00 50.0 17.00 6.00 46.00 11/12/2003 10/31/2008 Government of Chad Loan/ Credit APL 2 20.00 40.0 30.00 0 50.00 07/15/2007 06/30/2012 Government of Chad Loan/ Credit Public Disclosure Authorized APL 3 20.00 33.3 40.00 0 60.00 03/15/2011 12/31/2015 Government of Chad Loan/ Credit Total 63.00 93.00 156.00 1 [ ] Loan [X] Credit [X] Grant [ ] Guarantee [ ] Other: APL2 and APL3 IDA amounts are indicative. -

WAR and PROTECTED AREAS AREAS and PROTECTED WAR Vol 14 No 1 Vol 14 Protected Areas Programme Areas Protected
Protected Areas Programme Protected Areas Programme Vol 14 No 1 WAR AND PROTECTED AREAS 2004 Vol 14 No 1 WAR AND PROTECTED AREAS 2004 Parks Protected Areas Programme © 2004 IUCN, Gland, Switzerland Vol 14 No 1 WAR AND PROTECTED AREAS 2004 ISSN: 0960-233X Vol 14 No 1 WAR AND PROTECTED AREAS CONTENTS Editorial JEFFREY A. MCNEELY 1 Parks in the crossfire: strategies for effective conservation in areas of armed conflict JUDY OGLETHORPE, JAMES SHAMBAUGH AND REBECCA KORMOS 2 Supporting protected areas in a time of political turmoil: the case of World Heritage 2004 Sites in the Democratic Republic of Congo GUY DEBONNET AND KES HILLMAN-SMITH 9 Status of the Comoé National Park, Côte d’Ivoire and the effects of war FRAUKE FISCHER 17 Recovering from conflict: the case of Dinder and other national parks in Sudan WOUTER VAN HOVEN AND MUTASIM BASHIR NIMIR 26 Threats to Nepal’s protected areas PRALAD YONZON 35 Tayrona National Park, Colombia: international support for conflict resolution through tourism JENS BRÜGGEMANN AND EDGAR EMILIO RODRÍGUEZ 40 Establishing a transboundary peace park in the demilitarized zone on the Kuwaiti/Iraqi borders FOZIA ALSDIRAWI AND MUNA FARAJ 48 Résumés/Resumenes 56 Subscription/advertising details inside back cover Protected Areas Programme Vol 14 No 1 WAR AND PROTECTED AREAS 2004 ■ Each issue of Parks addresses a particular theme, in 2004 these are: Vol 14 No 1: War and protected areas Vol 14 No 2: Durban World Parks Congress Vol 14 No 3: Global change and protected areas ■ Parks is the leading global forum for information on issues relating to protected area establishment and management ■ Parks puts protected areas at the forefront of contemporary environmental issues, such as biodiversity conservation and ecologically The international journal for protected area managers sustainable development ISSN: 0960-233X Published three times a year by the World Commission on Protected Areas (WCPA) of IUCN – Subscribing to Parks The World Conservation Union. -

Status, Trends and Future Dynamics of Biodiversity and Ecosystems Underpinning Nature’S Contributions to People 1
CHAPTER 3 . STATUS, TRENDS AND FUTURE DYNAMICS OF BIODIVERSITY AND ECOSYSTEMS UNDERPINNING NATURE’S CONTRIBUTIONS TO PEOPLE 1 CHAPTER 2 CHAPTER 3 STATUS, TRENDS AND FUTURE DYNAMICS CHAPTER OF BIODIVERSITY AND 3 ECOSYSTEMS UNDERPINNING NATURE’S CONTRIBUTIONS CHAPTER TO PEOPLE 4 Coordinating Lead Authors Review Editors: Marie-Christine Cormier-Salem (France), Jonas Ngouhouo-Poufoun (Cameroon) Amy E. Dunham (United States of America), Christopher Gordon (Ghana) 3 CHAPTER This chapter should be cited as: Cormier-Salem, M-C., Dunham, A. E., Lead Authors Gordon, C., Belhabib, D., Bennas, N., Dyhia Belhabib (Canada), Nard Bennas Duminil, J., Egoh, B. N., Mohamed- (Morocco), Jérôme Duminil (France), Elahamer, A. E., Moise, B. F. E., Gillson, L., 5 Benis N. Egoh (Cameroon), Aisha Elfaki Haddane, B., Mensah, A., Mourad, A., Mohamed Elahamer (Sudan), Bakwo Fils Randrianasolo, H., Razaindratsima, O. H., Eric Moise (Cameroon), Lindsey Gillson Taleb, M. S., Shemdoe, R., Dowo, G., (United Kingdom), Brahim Haddane Amekugbe, M., Burgess, N., Foden, W., (Morocco), Adelina Mensah (Ghana), Ahmim Niskanen, L., Mentzel, C., Njabo, K. Y., CHAPTER Mourad (Algeria), Harison Randrianasolo Maoela, M. A., Marchant, R., Walters, M., (Madagascar), Onja H. Razaindratsima and Yao, A. C. Chapter 3: Status, trends (Madagascar), Mohammed Sghir Taleb and future dynamics of biodiversity (Morocco), Riziki Shemdoe (Tanzania) and ecosystems underpinning nature’s 6 contributions to people. In IPBES (2018): Fellow: The IPBES regional assessment report on biodiversity and ecosystem services for Gregory Dowo (Zimbabwe) Africa. Archer, E., Dziba, L., Mulongoy, K. J., Maoela, M. A., and Walters, M. (eds.). CHAPTER Contributing Authors: Secretariat of the Intergovernmental Millicent Amekugbe (Ghana), Neil Burgess Science-Policy Platform on Biodiversity (United Kingdom), Wendy Foden (South and Ecosystem Services, Bonn, Germany, Africa), Leo Niskanen (Finland), Christine pp. -

Reproductive Seasonality in Captive Wild Ruminants: Implications for Biogeographical Adaptation, Photoperiodic Control, and Life History
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2012 Reproductive seasonality in captive wild ruminants: implications for biogeographical adaptation, photoperiodic control, and life history Zerbe, Philipp Abstract: Zur quantitativen Beschreibung der Reproduktionsmuster wurden Daten von 110 Wildwiederkäuer- arten aus Zoos der gemässigten Zone verwendet (dabei wurde die Anzahl Tage, an denen 80% aller Geburten stattfanden, als Geburtenpeak-Breite [BPB] definiert). Diese Muster wurden mit verschiede- nen biologischen Charakteristika verknüpft und mit denen von freilebenden Tieren verglichen. Der Bre- itengrad des natürlichen Verbreitungsgebietes korreliert stark mit dem in Menschenobhut beobachteten BPB. Nur 11% der Spezies wechselten ihr reproduktives Muster zwischen Wildnis und Gefangenschaft, wobei für saisonale Spezies die errechnete Tageslichtlänge zum Zeitpunkt der Konzeption für freilebende und in Menschenobhut gehaltene Populationen gleich war. Reproduktive Saisonalität erklärt zusätzliche Varianzen im Verhältnis von Körpergewicht und Tragzeit, wobei saisonalere Spezies für ihr Körpergewicht eine kürzere Tragzeit aufweisen. Rückschliessend ist festzuhalten, dass Photoperiodik, speziell die abso- lute Tageslichtlänge, genetisch fixierter Auslöser für die Fortpflanzung ist, und dass die Plastizität der Tragzeit unterstützend auf die erfolgreiche Verbreitung der Wiederkäuer in höheren Breitengraden wirkte. A dataset on 110 wild ruminant species kept in captivity in temperate-zone zoos was used to describe their reproductive patterns quantitatively (determining the birth peak breadth BPB as the number of days in which 80% of all births occur); then this pattern was linked to various biological characteristics, and compared with free-ranging animals. Globally, latitude of natural origin highly correlates with BPB observed in captivity, with species being more seasonal originating from higher latitudes. -

Slender-Horned Gazelle Gazella Leptoceros Conservation Strategy 2020-2029
Slender-horned Gazelle Gazella leptoceros Conservation Strategy 2020-2029 Slender-horned Gazelle (Gazella leptoceros) Slender-horned Gazelle (:Conservation Strategy 2020-2029 Gazella leptoceros ) :Conservation Strategy 2020-2029 Conservation Strategy for the Slender-horned Gazelle Conservation Strategy for the Slender-horned Conservation Strategy for the Slender-horned The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of any participating organisation concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organisations. Compiled and edited by David Mallon, Violeta Barrios and Helen Senn Contributors Teresa Abaígar, Abdelkader Benkheira, Roseline Beudels-Jamar, Koen De Smet, Husam Elalqamy, Adam Eyres, Amina Fellous-Djardini, Héla Guidara-Salman, Sander Hofman, Abdelkader Jebali, Ilham Kabouya-Loucif, Maher Mahjoub, Renata Molcanova, Catherine Numa, Marie Petretto, Brigid Randle, Tim Wacher Published by IUCN SSC Antelope Specialist Group and Royal Zoological Society of Scotland, Edinburgh, United Kingdom Copyright ©2020 IUCN SSC Antelope Specialist Group Reproduction of this publication for educational or other non-commercial purposes is authorised without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Recommended citation IUCN SSC ASG and RZSS. 2020. Slender-horned Gazelle (Gazella leptoceros): Conservation strategy 2020-2029. IUCN SSC Antelope Specialist Group and Royal Zoological Society of Scotland. -

Detection of Babesia Spp. in Free-Ranging Pukus, Kobus Vardonii, on a Game Ranch in Zambia
ISSN (Print) 0023-4001 ISSN (Online) 1738-0006 Korean J Parasitol Vol. 49, No. 4: 437-440, December 2011 ▣ BRIEF COMMUNICATION http://dx.doi.org/10.3347/kjp.2011.49.4.437 Detection of Babesia spp. in Free-Ranging Pukus, Kobus vardonii, on a Game Ranch in Zambia 1, 2 2 3 Hetron Mweemba Munang’andu *, Musso Munyeme , Andrew Mubila Nambota , King Shimumbo Nalubamba and Victor M. Siamudaala4 1Norwegian School of Veterinary Sciences, Department of Basic Sciences and Aquatic Medicine, Section of Aquatic Medicine and Nutrition, P.O. Box 8146 Dep NO-033, Oslo, Norway; 2Department of Disease Control, School of Veterinary Medicine, University of Zambia, P.O. Box 32379, Lusaka, Zambia; 3Department of Clinical Studies, School of Veterinary Medicine, University of Zambia, P.O. Box 32379, Lusaka, Zambia; 4Kavango Transfrontier Wild Animal, Gaborone, Botswana Abstract: Babesia spp. were detected from 4 asymptomatic pukus captured on a game ranch in central Zambia in Octo- ber 2008. Blood smears were examined in 4 species of aymptomatic free-ranging antelopes, namely the puku (Kobus vor danii), reedbuck (Redunca arundinum), bushbuck (Tragelaphus sylvaticus), and kudu (Tragelaphus strepsiceros), and showed the presence of Babesia parasites only in the puku. In the puku, the prevalence of babesiosis was estimated at 33.3% (n=12), while the overall prevalence in all examined animals was 8.5% (n=47). The parasites showed morphologi- cal characteristics of paired ring-like stages with the length varying between 1.61 µm and 3.02 µm (mean=2.12 µm, n= 27; SD=0.76 µm). Both the infected and non-infected pukus showed good body condition scores (BCS), while the domi- nant tick species detected from all animals were Rhipicephalus appendiculatus, Rhipicephalus spp., and Boophilus spp. -

Caribou (Barren-Ground Population) Rangifer Tarandus
COSEWIC Assessment and Status Report on the Caribou Rangifer tarandus Barren-ground population in Canada THREATENED 2016 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2016. COSEWIC assessment and status report on the Caribou Rangifer tarandus, Barren-ground population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 123 pp. (http://www.registrelep-sararegistry.gc.ca/default.asp?lang=en&n=24F7211B-1). Production note: COSEWIC would like to acknowledge Anne Gunn, Kim Poole, and Don Russell for writing the status report on Caribou (Rangifer tarandus), Barren-ground population, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Justina Ray, Co-chair of the COSEWIC Terrestrial Mammals Specialist Subcommittee, with the support of the members of the Terrestrial Mammals Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment and Climate Change Canada Ottawa, ON K1A 0H3 Tel.: 819-938-4125 Fax: 819-938-3984 E-mail: [email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Caribou (Rangifer tarandus), population de la toundra, au Canada. Cover illustration/photo: Caribou — Photo by A. Gunn. Her Majesty the Queen in Right of Canada, 2016. Catalogue No. CW69-14/746-2017E-PDF ISBN 978-0-660-07782-6 COSEWIC Assessment Summary Assessment Summary – November 2016 Common name Caribou - Barren-ground population Scientific name Rangifer tarandus Status Threatened Reason for designation Members of this population give birth on the open arctic tundra, and most subpopulations (herds) winter in vast subarctic forests. -

Gallina & Mandujano
Mongabay.com Open Access Journal - Tropical Conservation Science Vol. 2 (2):116-127, 2009 Special issue: introduction Research on ecology, conservation and management of wild ungulates in Mexico Sonia Gallina1 and Salvador Mandujano1 1 Departamento de Biodiversidad y Ecología Animal, Instituto de Ecología A. C., km. 2.5 Carret. Ant. Coatepec No. 351, Congregación del Haya, Xalapa 91070, Ver. México. E‐mail: <[email protected]>; <[email protected]> Abstract This special issue of Tropical Conservation Science provides a synopsis of nine of the eleven presentations on ungulates presented at the Symposium on Ecology and Conservation of Ungulates in Mexico during the Mexican Congress of Ecology held in November 2008 in Merida, Yucatan. Of the eleven species of wild ungulates in Mexico (Baird´s tapir Tapirus bairdii, pronghorn antelope Antilocapra americana, American bison Bison bison, bighorn sheep Ovis canadensis, elk Cervus canadensis, red brocket deer Mazama temama, Yucatan brown brocket Mazama pandora, mule deer Odocoileus hemionus, white-tailed deer Odocoileus virginianus, white-lipped peccary Tayassu pecari and collared peccary Pecari tajacu), studies which concern four of these species are presented: Baird’s tapir and the white lipped peccary, which are tropical species in danger of extinction; the bighorn sheep, of high value for hunting in the north-west; and the white-tailed deer, the most studied ungulate in Mexico due to its wide distribution in the country and high hunting and cultural value. In addition, two studies of exotic species, wild boar (Sus scrofa) and red deer (Cervus elaphus), are presented. Issues addressed in these studies are: population estimates, habitat use, evaluation of UMA (Spanish acronym for ‘Wildlife Conservation, Management and Sustainable Utilization Units’) and ANP (Spanish acronym for ‘Natural Protected Areas’) to sustain minimum viable populations, and the effect of alien species in protected areas and UMA, all of which allow an insight into ungulate conservation and management within the country.