Gazella Leptoceros
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Download File
Italy and the Sanusiyya: Negotiating Authority in Colonial Libya, 1911-1931 Eileen Ryan Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2012 ©2012 Eileen Ryan All rights reserved ABSTRACT Italy and the Sanusiyya: Negotiating Authority in Colonial Libya, 1911-1931 By Eileen Ryan In the first decade of their occupation of the former Ottoman territories of Tripolitania and Cyrenaica in current-day Libya, the Italian colonial administration established a system of indirect rule in the Cyrenaican town of Ajedabiya under the leadership of Idris al-Sanusi, a leading member of the Sufi order of the Sanusiyya and later the first monarch of the independent Kingdom of Libya after the Second World War. Post-colonial historiography of modern Libya depicted the Sanusiyya as nationalist leaders of an anti-colonial rebellion as a source of legitimacy for the Sanusi monarchy. Since Qaddafi’s revolutionary coup in 1969, the Sanusiyya all but disappeared from Libyan historiography as a generation of scholars, eager to fill in the gaps left by the previous myopic focus on Sanusi elites, looked for alternative narratives of resistance to the Italian occupation and alternative origins for the Libyan nation in its colonial and pre-colonial past. Their work contributed to a wider variety of perspectives in our understanding of Libya’s modern history, but the persistent focus on histories of resistance to the Italian occupation has missed an opportunity to explore the ways in which the Italian colonial framework shaped the development of a religious and political authority in Cyrenaica with lasting implications for the Libyan nation. -

Assessment of the Physico-Chemical and Biological Quality of Surface Waters in Arid and Semi-Arid Regions of Algeria (North-Africa)
Bull. Soc. zool. Fr., 2019, 144(4) : 157-178. Hydrobiologie ASSESSMENT OF THE PHYSICO-CHEMICAL AND BIOLOGICAL QUALITY OF SURFACE WATERS IN ARID AND SEMI-ARID REGIONS OF ALGERIA (NORTH-AFRICA) par Nassima SELLAM 1,4,*, Amador VIÑOLAS 2, Fatah ZOUGGAGHE 3,4 & Riadh MOULAÏ 4 An assessment of the physico-chemical and the biological quality of surface waters through the use of macroinvertebrates as bioindicators was conducted in two rivers in Algeria, located in semi-arid and arid regions. These are Wadi M’zi (Laghouat region) and Wadi Djedir (Djelfa region). The sampling strategy developed in this work is based on the analysis of the upstream and downstream waters of each watercourse. - 2- - 2+ Eleven physico-chemical parameters (T°C, pH, CE, OD, Cl , SO4 , NO3 , Salinity, Ca , 2+ - Mg and HCO3 ) were measured to establish a diagnosis of the state of health of these aquatic ecosystems. Macroinvertebrates were studied using kick-net sampling at eight study sites. The faunistic inventory of benthic macroinvertebrates identified 37 families, mostly represented by insect larvae (96%). Among these, Diptera and Ephemeroptera were the most dominant orders, with other groups being relatively poorly represented. The results obtained show that the physico-chemical quality of the water is slightly dete- riorated, but the quality is still evaluated as ‘good’ according to the biotic indices 1. Université d’Amar Thelidji. Faculté des Sciences. Département de Biologie. 03000 Laghouat. Algérie. 2. Museu de Ciències Naturals de Barcelona. Laboratori de Natura. Collecció d’artròpodes. Passeig Picasso s/n 08003 Barcelona. Catalunya. 3. Université AMO de Bouira. Faculté des Sciences de la Nature et la Vie et Sciences de la Terre. -
Summary of Protected Areas in Chad
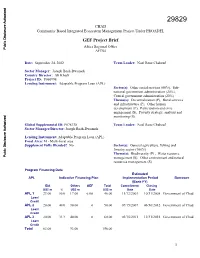
CHAD Community Based Integrated Ecosystem Management Project Under PROADEL GEF Project Brief Africa Regional Office Public Disclosure Authorized AFTS4 Date: September 24, 2002 Team Leader: Noel Rene Chabeuf Sector Manager: Joseph Baah-Dwomoh Country Director: Ali Khadr Project ID: P066998 Lending Instrument: Adaptable Program Loan (APL) Sector(s): Other social services (60%), Sub- national government administration (20%), Central government administration (20%) Theme(s): Decentralization (P), Rural services Public Disclosure Authorized and infrastructure (P), Other human development (P), Participation and civic engagement (S), Poverty strategy, analysis and monitoring (S) Global Supplemental ID: P078138 Team Leader: Noel Rene Chabeuf Sector Manager/Director: Joseph Baah-Dwomoh Lending Instrument: Adaptable Program Loan (APL) Focal Area: M - Multi-focal area Supplement Fully Blended? No Sector(s): General agriculture, fishing and forestry sector (100%) Theme(s): Biodiversity (P) , Water resource Public Disclosure Authorized management (S), Other environment and natural resources management (S) Program Financing Data Estimated APL Indicative Financing Plan Implementation Period Borrower (Bank FY) IDA Others GEF Total Commitment Closing US$ m % US$ m US$ m Date Date APL 1 23.00 50.0 17.00 6.00 46.00 11/12/2003 10/31/2008 Government of Chad Loan/ Credit APL 2 20.00 40.0 30.00 0 50.00 07/15/2007 06/30/2012 Government of Chad Loan/ Credit Public Disclosure Authorized APL 3 20.00 33.3 40.00 0 60.00 03/15/2011 12/31/2015 Government of Chad Loan/ Credit Total 63.00 93.00 156.00 1 [ ] Loan [X] Credit [X] Grant [ ] Guarantee [ ] Other: APL2 and APL3 IDA amounts are indicative. -

Cuvier's Gazelle in Algeria
Cuvier's gazelle in Algeria Koen de Smet Cuvier's gazelle Gazella cuvieri is endemic to North Africa and has been classified as Endangered by IUCN. Algeria holds most of the remnant population but little is known about its current status and distribution there. The author studied the gazelle between 1984 and 1988 and found that while populations have declined in some areas, at least 560 individuals survive, some in protected areas. Introduction where gazelles had been reported by a nation- al survey undertaken by the Algerian Forest The edmi or Cuvier's gazelle Gazella cuvieri is Service in 1983 or by other reliable persons. In a North African endemic, with its distribution all cases the habitat was described, the area limited to Morocco (including the Western searched for tracks and latrines, and local peo- Sahara), Algeria and Tunisia (Figure 1), ple were questioned to gain some idea of although De Beaux (1928) and Hufnagl (1980) group size and habits of the animals. believed that it also once existed in north-east- Furthermore, the regions where no informa- ern Libya. We knew very little about the tion was available but which were situated species in Algeria; it was considered to be within the geographical and ecological limits rare, but no information was available about of the species, were explored. Maps of north its numbers. The IUCN (1974) classified the Algeria were also examined for localities species as Endangered and believed that whose names referred to gazelles. Algeria held most of the remnant population. In addition, almost nothing was known about its whereabouts and its habitat preferences. -

Status, Trends and Future Dynamics of Biodiversity and Ecosystems Underpinning Nature’S Contributions to People 1
CHAPTER 3 . STATUS, TRENDS AND FUTURE DYNAMICS OF BIODIVERSITY AND ECOSYSTEMS UNDERPINNING NATURE’S CONTRIBUTIONS TO PEOPLE 1 CHAPTER 2 CHAPTER 3 STATUS, TRENDS AND FUTURE DYNAMICS CHAPTER OF BIODIVERSITY AND 3 ECOSYSTEMS UNDERPINNING NATURE’S CONTRIBUTIONS CHAPTER TO PEOPLE 4 Coordinating Lead Authors Review Editors: Marie-Christine Cormier-Salem (France), Jonas Ngouhouo-Poufoun (Cameroon) Amy E. Dunham (United States of America), Christopher Gordon (Ghana) 3 CHAPTER This chapter should be cited as: Cormier-Salem, M-C., Dunham, A. E., Lead Authors Gordon, C., Belhabib, D., Bennas, N., Dyhia Belhabib (Canada), Nard Bennas Duminil, J., Egoh, B. N., Mohamed- (Morocco), Jérôme Duminil (France), Elahamer, A. E., Moise, B. F. E., Gillson, L., 5 Benis N. Egoh (Cameroon), Aisha Elfaki Haddane, B., Mensah, A., Mourad, A., Mohamed Elahamer (Sudan), Bakwo Fils Randrianasolo, H., Razaindratsima, O. H., Eric Moise (Cameroon), Lindsey Gillson Taleb, M. S., Shemdoe, R., Dowo, G., (United Kingdom), Brahim Haddane Amekugbe, M., Burgess, N., Foden, W., (Morocco), Adelina Mensah (Ghana), Ahmim Niskanen, L., Mentzel, C., Njabo, K. Y., CHAPTER Mourad (Algeria), Harison Randrianasolo Maoela, M. A., Marchant, R., Walters, M., (Madagascar), Onja H. Razaindratsima and Yao, A. C. Chapter 3: Status, trends (Madagascar), Mohammed Sghir Taleb and future dynamics of biodiversity (Morocco), Riziki Shemdoe (Tanzania) and ecosystems underpinning nature’s 6 contributions to people. In IPBES (2018): Fellow: The IPBES regional assessment report on biodiversity and ecosystem services for Gregory Dowo (Zimbabwe) Africa. Archer, E., Dziba, L., Mulongoy, K. J., Maoela, M. A., and Walters, M. (eds.). CHAPTER Contributing Authors: Secretariat of the Intergovernmental Millicent Amekugbe (Ghana), Neil Burgess Science-Policy Platform on Biodiversity (United Kingdom), Wendy Foden (South and Ecosystem Services, Bonn, Germany, Africa), Leo Niskanen (Finland), Christine pp. -

Scf Pan Sahara Wildlife Survey
SCF PAN SAHARA WILDLIFE SURVEY PSWS Technical Report 12 SUMMARY OF RESULTS AND ACHIEVEMENTS OF THE PILOT PHASE OF THE PAN SAHARA WILDLIFE SURVEY 2009-2012 November 2012 Dr Tim Wacher & Mr John Newby REPORT TITLE Wacher, T. & Newby, J. 2012. Summary of results and achievements of the Pilot Phase of the Pan Sahara Wildlife Survey 2009-2012. SCF PSWS Technical Report 12. Sahara Conservation Fund. ii + 26 pp. + Annexes. AUTHORS Dr Tim Wacher (SCF/Pan Sahara Wildlife Survey & Zoological Society of London) Mr John Newby (Sahara Conservation Fund) COVER PICTURE New-born dorcas gazelle in the Ouadi Rimé-Ouadi Achim Game Reserve, Chad. Photo credit: Tim Wacher/ZSL. SPONSORS AND PARTNERS Funding and support for the work described in this report was provided by: • His Highness Sheikh Mohammed bin Zayed Al Nahyan, Crown Prince of Abu Dhabi • Emirates Center for Wildlife Propagation (ECWP) • International Fund for Houbara Conservation (IFHC) • Sahara Conservation Fund (SCF) • Zoological Society of London (ZSL) • Ministère de l’Environnement et de la Lutte Contre la Désertification (Niger) • Ministère de l’Environnement et des Ressources Halieutiques (Chad) • Direction de la Chasse, Faune et Aires Protégées (Niger) • Direction des Parcs Nationaux, Réserves de Faune et de la Chasse (Chad) • Direction Générale des Forêts (Tunis) • Projet Antilopes Sahélo-Sahariennes (Niger) ACKNOWLEDGEMENTS The Sahara Conservation Fund sincerely thanks HH Sheikh Mohamed bin Zayed Al Nahyan, Crown Prince of Abu Dhabi, for his interest and generosity in funding the Pan Sahara Wildlife Survey through the Emirates Centre for Wildlife Propagation (ECWP) and the International Fund for Houbara Conservation (IFHC). This project is carried out in association with the Zoological Society of London (ZSL). -

Slender-Horned Gazelle Gazella Leptoceros Conservation Strategy 2020-2029
Slender-horned Gazelle Gazella leptoceros Conservation Strategy 2020-2029 Slender-horned Gazelle (Gazella leptoceros) Slender-horned Gazelle (:Conservation Strategy 2020-2029 Gazella leptoceros ) :Conservation Strategy 2020-2029 Conservation Strategy for the Slender-horned Gazelle Conservation Strategy for the Slender-horned Conservation Strategy for the Slender-horned The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of any participating organisation concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organisations. Compiled and edited by David Mallon, Violeta Barrios and Helen Senn Contributors Teresa Abaígar, Abdelkader Benkheira, Roseline Beudels-Jamar, Koen De Smet, Husam Elalqamy, Adam Eyres, Amina Fellous-Djardini, Héla Guidara-Salman, Sander Hofman, Abdelkader Jebali, Ilham Kabouya-Loucif, Maher Mahjoub, Renata Molcanova, Catherine Numa, Marie Petretto, Brigid Randle, Tim Wacher Published by IUCN SSC Antelope Specialist Group and Royal Zoological Society of Scotland, Edinburgh, United Kingdom Copyright ©2020 IUCN SSC Antelope Specialist Group Reproduction of this publication for educational or other non-commercial purposes is authorised without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Recommended citation IUCN SSC ASG and RZSS. 2020. Slender-horned Gazelle (Gazella leptoceros): Conservation strategy 2020-2029. IUCN SSC Antelope Specialist Group and Royal Zoological Society of Scotland. -

The Continental Intercalaire Aquifer at the Kébili Geothermal Field, Southern Tunisia
Proceedings World Geothermal Congress 2005 Antalya, Turkey, 24-29 April 2005 The Continental Intercalaire Aquifer at the Kébili Geothermal Field, Southern Tunisia Aissa Agoun Regional Commissariat for Agricultural Development Water Resources Departement, C.R.D.A Kébili, Kébili 4200, TUNISIA [email protected] Keywords: CI, Kébili, wells, artesian wellheads like valves and monitoring points. Also drawdown of the water level has been observed due to the ABSTRACT increasing water demands. Radiocarbon analysis has shown that radiocarbon is present at between 2 and 10 pmc which The C.I. "Continental Intercalaire" aquifer is an extensive leads to the conclusion that the water is recharged during horizontal sandstone reservoir (Neocomien: Lower the late Pleistocene 25,000 years B.P, corresponding to the Cretaceous: Purbecko-Wealdien). The CI is one of the last glaciation (Edmonds et al., 1997). Water is fossil and largest aquifers in the world covering more than 1 million 2 without actual recharge, so to preserve the trans-borderers km in Tunisia, Algeria and Libya. This aquifer covers reservoir, more research must be carried out on the aquifer. 80,000 km2 in Tunisia Full collaboration with Algerian and Libyan organizations In Kébili field in southern Tunisia, the geothermal water is is necessary in order to achieve economical and sustainable about 25 to 50 thousand years old and of sulphate chlorite future production and regional development. Long term type. The depth of the reservoir ranges from 1000 to 2800 monitoring of pressure temperature and salinity at the m. wellhead for each production well should be carried out. Any increase of drawdown during the next 20 years caused The piézométric level is about 200 meters above the by increased production will lead to environmental changes. -

The Human Conveyor Belt : Trends in Human Trafficking and Smuggling in Post-Revolution Libya
The Human Conveyor Belt : trends in human trafficking and smuggling in post-revolution Libya March 2017 A NETWORK TO COUNTER NETWORKS The Human Conveyor Belt : trends in human trafficking and smuggling in post-revolution Libya Mark Micallef March 2017 Cover image: © Robert Young Pelton © 2017 Global Initiative against Transnational Organized Crime. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without permission in writing from the Global Initiative. Please direct inquiries to: The Global Initiative against Transnational Organized Crime WMO Building, 2nd Floor 7bis, Avenue de la Paix CH-1211 Geneva 1 Switzerland www.GlobalInitiative.net Acknowledgments This report was authored by Mark Micallef for the Global Initiative, edited by Tuesday Reitano and Laura Adal. Graphics and layout were prepared by Sharon Wilson at Emerge Creative. Editorial support was provided by Iris Oustinoff. Both the monitoring and the fieldwork supporting this document would not have been possible without a group of Libyan collaborators who we cannot name for their security, but to whom we would like to offer the most profound thanks. The author is also thankful for comments and feedback from MENA researcher Jalal Harchaoui. The research for this report was carried out in collaboration with Migrant Report and made possible with funding provided by the Ministry of Foreign Affairs of Norway, and benefitted from synergies with projects undertaken by the Global Initiative in partnership with the Institute for Security Studies and the Hanns Seidel Foundation, the United Nations University, and the UK Department for International Development. About the Author Mark Micallef is an investigative journalist and researcher specialised on human smuggling and trafficking. -

MPLS VPN Service
MPLS VPN Service PCCW Global’s MPLS VPN Service provides reliable and secure access to your network from anywhere in the world. This technology-independent solution enables you to handle a multitude of tasks ranging from mission-critical Enterprise Resource Planning (ERP), Customer Relationship Management (CRM), quality videoconferencing and Voice-over-IP (VoIP) to convenient email and web-based applications while addressing traditional network problems relating to speed, scalability, Quality of Service (QoS) management and traffic engineering. MPLS VPN enables routers to tag and forward incoming packets based on their class of service specification and allows you to run voice communications, video, and IT applications separately via a single connection and create faster and smoother pathways by simplifying traffic flow. Independent of other VPNs, your network enjoys a level of security equivalent to that provided by frame relay and ATM. Network diagram Database Customer Portal 24/7 online customer portal CE Router Voice Voice Regional LAN Headquarters Headquarters Data LAN Data LAN Country A LAN Country B PE CE Customer Router Service Portal PE Router Router • Router report IPSec • Traffic report Backup • QoS report PCCW Global • Application report MPLS Core Network Internet IPSec MPLS Gateway Partner Network PE Router CE Remote Router Site Access PE Router Voice CE Voice LAN Router Branch Office CE Data Branch Router Office LAN Country D Data LAN Country C Key benefits to your business n A fully-scalable solution requiring minimal investment -

586 PROTECTION of ENDANGERED SPECIES of ANIMALS and PLANTS ORDINANCE Gazette Number Version Date Long Title LN 206 Of
Chapter: 586 PROTECTION OF ENDANGERED SPECIES OF Gazette Number Version Date ANIMALS AND PLANTS ORDINANCE Long title L.N. 206 of 2006 01/12/2006 An Ordinance to give effect in Hong Kong to the Convention on International Trade in Endangered Species of Wild Fauna and Flora signed in Washington D.C. on 3 March 1973; to regulate the import, introduction from the sea, export, re-export, and possession or control of certain endangered species of animals and plants and parts and derivatives of those species; and to provide for incidental and connected matters. [1 December 2006] L.N. 206 of 2006 (Originally 3 of 2006) Part: 1 PRELIMINARY L.N. 206 of 2006 01/12/2006 Section: 1 Short title L.N. 206 of 2006 01/12/2006 (1) This Ordinance may be cited as the Protection of Endangered Species of Animals and Plants Ordinance. (2) (Omitted as spent) Section: 2 Interpretation L.N. 130 of 2007 01/07/2007 Remarks: For the saving and transitional provisions relating to the amendments made by the Resolution of the Legislative Council (L.N. 130 of 2007), see paragraph (12) of that Resolution. (1) In this Ordinance, unless the context otherwise requires— "advertisement" (廣告), in relation to a specimen of a scheduled species, means any form of advertising that describes, makes reference or alludes in any other way to that scheduled species or specimen— (a) whether directly or indirectly; (b) whether orally, in writing in any language, diagrammatically, pictorially, by the use of symbols or photographs, or in any combination of them; and (c) whether or not the -

Gazella Dorcas) Using Distance Sampling in Southern Sinai, EGYPT
DEVELOPING AND ASSESSING A POPULATION MONITORING PROGRAM FOR DORCAS GAZELLE (GAZELLA DORCAS) USING DISTANCE SAMPLING IN SOUTHERN SINAI, EGYPT Husam E. M. El Alqamy A Thesis Submitted for the Degree of MPhil at the University of St Andrews 2003 Full metadata for this item is available in Research@StAndrews:FullText at: http://research-repository.st-andrews.ac.uk/ Please use this identifier to cite or link to this item: http://hdl.handle.net/10023/5912 This item is protected by original copyright Developing and Assessing a Population Monitoring Program for Dorcas Gazelle (Gazella dorcas) Using Distance Sampling in Southern Sinai, EGYPT Husam E. M. El Alqamy Thesis submitted for the degree of MASTER OF PHILOSOPHY In the School of Biology Division of Environmental & Evolutionary Biology UNIVERSITY OF ST. ANDREWS August 2002 i Abstract ...................................................................................................... i Chapter 1 ................................................................................................... 1 Introduction .............................................................................................. 1 1.1 Conservation Legislation in Egypt: A Background..................... 1 1.2 General Ecology of St. Katherine Protectorate ........................... 1 1.3 Aims of Present Work .................................................................... 2 1.4 Identification and Description of Dorcas Gazelle ........................ 3 1.5 Taxonomic Status of Gazelles in Sinai .........................................