The Functional Significance of Panting As a Mechanism of Thermoregulation and Its Relationship to the Critical Thermal Maxima in Lizards Caleb L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Life History of the Coppertail Skink (Ctenotus Taeniolatus) in Southeastern Australia
Herpetological Conservation and Biology 15(2):409–415. Submitted: 11 February 2020; Accepted: 19 May 2020; Published: 31 August 2020. LIFE HISTORY OF THE COPPERTAIL SKINK (CTENOTUS TAENIOLATUS) IN SOUTHEASTERN AUSTRALIA DAVID A. PIKE1,2,6, ELIZABETH A. ROZNIK3, JONATHAN K. WEBB4, AND RICHARD SHINE1,5 1School of Biological Sciences A08, University of Sydney, New South Wales 2006, Australia 2Present address: Department of Biology, Rhodes College, Memphis, Tennessee 38112, USA 3Department of Conservation and Research, Memphis Zoo, Memphis, Tennessee 38112, USA 4School of Life Sciences, University of Technology Sydney, Broadway, New South Wales 2007, Australia 5Present address: Department of Biological Sciences, Macquarie University, New South Wales 2109, Australia 6Corresponding author, e-mail: [email protected] Abstract.—The global decline of reptiles is a serious problem, but we still know little about the life histories of most species, making it difficult to predict which species are most vulnerable to environmental change and why they may be vulnerable. Life history can help dictate resilience in the face of decline, and therefore understanding attributes such as sexual size dimorphism, site fidelity, and survival rates are essential. Australia is well-known for its diversity of scincid lizards, but we have little detailed knowledge of the life histories of individual scincid species. To examine the life history of the Coppertail Skink (Ctenotus taeniolatus), which uses scattered surface rocks as shelter, we estimated survival rates, growth rates, and age at maturity during a three-year capture-mark- recapture study. We captured mostly females (> 84%), and of individuals captured more than once, we captured 54.3% at least twice beneath the same rock, and of those, 64% were always beneath the same rock (up to five captures). -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Animal Health Requirements for Importation of Reptiles, Amphibians, and Invertebrates Into Denmark
INTERNATIONAL TRADE DIVISION ANIMAL HEALTH REQUIREMENTS FOR IMPORTATION OF REPTILES, AMPHIBIANS, AND INVERTEBRATES INTO DENMARK. La 23,0-2100 These animal health requirements concern veterinary import requirements and certification re- quirements alone and shall apply without prejudice to other Danish and EU legislation. Reptiles, amphibians and invertebrates meaning animals of the Family/Species listed below (please note the exceptions): Order Family/Species Crocodilia Ostaeolaemus spp. (Dwarf Crocodile), Paleosuchus spp. (Cuvier's Dwarf Caiman and Smooth-fronted (Crocodiles) Caiman) and Alligator sinensis (Chinese alligator) Rhynchocephalia Sphenodontidae (Tuataras) Squamata (Liz- Corytophanidae, Iguanidae , Phrynosomatidae, Polychrotidae, Tropiduridae, Crotaphytidae, Opluridae, ards and snakes) Hoplocercidae, Agamidae, Chamaeleonidae, Gekkonidae, Pygopodidae, Dibamidae, Scincidae, Cordy- lidae, Gerrhosauridae, Xantusiidae, Lacertidae, Teiidae, Gymnophthalmidae, Anguidae, Anniellidae, Xenosauridae, Varanidae (except Varanus komodoensis, Varanus salvator, Varanus salvadoiri, Vara- nus niloticus and Varanus ornatus), Lanthanotidae, Helodermatidae, Aniliidae, Anomochilidae, Boidae (except Eunectes murinus) , Bolyeriidae, Cylindrophiidae , Loxocemidae , Pythonidae (except Python molurus, Python sebae and Python reticulatus), Tropidophiidae , Uropeltidae, Xenopeltidae, Anomalepididae, Leptotyphlopidae, Typhlopidae, Acrochordidae, Atractaspididae (except Atractaspis spp. and Macrelaps spp.), Colubridae (except Thelotornis spp., Dispholidus -

[email protected] Biodiversity @Maddreptiles
Timothy Colston Biological Science Harnessing NGS Technologies to Understand Biological Diversification: From Microbes to Macroevolutionary Patterns [email protected] Biodiversity @maddreptiles Source: International Conference on Biodiversity Motivation & Tools –Molecular [email protected] @maddreptiles (NGS) [email protected] Biodiversity @maddreptiles Source: International Conference on Biodiversity [email protected] Biodiversity @maddreptiles [email protected] Biodiversity –the “microbiome” @maddreptiles NGS Sequencing [email protected] Biodiversity –the “microbiome” @maddreptiles NGS Sequencing [email protected] Biodiversity –the “microbiome” @maddreptiles • Plants and Animals are “metagenomic organisms” – Co‐evolution • Host‐associated microbial cells ~ 10X number of host cells – Fitness/Selection – Heritable by Gaby D'Allesandro / © AMNH [email protected] Biodiversity –the “microbiome” @maddreptiles • Plants and Animals are “metagenomic organisms” – Co‐evolution • Host‐associated microbial genes > 10X number of host cells – Fitness/Selection – Heritable by Gaby D'Allesandro / © AMNH [email protected] Biodiversity –the “microbiome” @maddreptiles Mammals Fish Birds Amphibians Reptiles Colston, T.J. & Jackson, C.R. (2016) Molecular Ecology The Reptile Microbiome C h a m A a g e a A l m e m V o L i a p d n a h a i r H d a n i s e e L t T a n b h S l a r a i A o e A d o a c h X e d n g n a n e i n D e T e o n g r e o n i n t n r r a d i u i L t i s m o o e d o c i a e i d a p p a l s t d e i a y l h o i e a u d a i a l d t C c i B o r u -

California Wildlife Habitat Relationships System California Department of Fish and Wildlife California Interagency Wildlife Task Group
California Wildlife Habitat Relationships System California Department of Fish and Wildlife California Interagency Wildlife Task Group BAJA CALIFORNIA COLLARED LIZARD Crotaphytus vestigium Family: CROTAPHYTIDAE Order: SQUAMATA Class: REPTILIA R093 Written by: T. Kucera, 1998 Updated by: CWHR Staff, February 2008 DISTRIBUTION, ABUNDANCE, AND SEASONALITY The Baja California collared lizard inhabits the eastern face of the peninsular ranges and adjacent rocky slopes from the northern slope of the San Jacinto mountains to the Mexican border and south into Baja California, Mexico (Sanborn and Loomis 1979, McGuire 1996). It is generally restricted to rocky outcroppings on more rugged portions of alluvial fans, desert hillsides, canyons, and lava flows. It is most common in xeric, rocky areas with little vegetation, including desert succulent shrub, desert scrub, and desert wash habitats. Little has been written about its natural history. The Baja California collared lizard is active in the spring and summer and to a lesser extent in the fall. SPECIFIC HABITAT REQUIREMENTS Feeding: Little is known of the diet of this species. The diet of the closely related Mojave black- collared lizard (C. bicinctores) consists largely of arthropods and small vertebrates (Stebbins 1985, McGuire 1996). Plant material is also occasionally consumed. Cover: This species prefers rocky areas and seeks cover under rocks and in cracks and crevices and rodent holes (Stebbins 1985), occasionally bounding bipedally from stone to stone when disturbed (McGuire 1996). Reproduction: Little is known about the reproductive requirements of this species. The closely related Mojave black-collared lizard lays eggs and presumably constructs its own nest but there are no reports. -

Pdf> (Accessed of Life
Molecular Phylogenetics and Evolution 94 (2016) 537–547 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species q ⇑ Yuchi Zheng a,b, John J. Wiens b, a Department of Herpetology, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China b Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721-088, USA article info abstract Article history: Two common approaches for estimating phylogenies in species-rich groups are to: (i) sample many loci Received 26 May 2015 for few species (e.g. phylogenomic approach), or (ii) sample many species for fewer loci (e.g. supermatrix Revised 30 September 2015 approach). In theory, these approaches can be combined to simultaneously resolve both higher-level rela- Accepted 8 October 2015 tionships (with many genes) and species-level relationships (with many taxa). However, fundamental Available online 22 October 2015 questions remain unanswered about this combined approach. First, will higher-level relationships more closely resemble those estimated from many genes or those from many taxa? Second, will branch sup- Keywords: port increase for higher-level relationships (relative to the estimate from many taxa)? Here, we address Missing data these questions in squamate reptiles. We combined two recently published datasets, one based on 44 Phylogenomic Phylogeny genes for 161 species, and one based on 12 genes for 4161 species. The likelihood-based tree from the Squamata combined matrix (52 genes, 4162 species) shared more higher-level clades with the 44-gene tree (90% Supermatrix vs. -

Standard Common and Current Scientific Names for North American Amphibians, Turtles, Reptiles & Crocodilians
STANDARD COMMON AND CURRENT SCIENTIFIC NAMES FOR NORTH AMERICAN AMPHIBIANS, TURTLES, REPTILES & CROCODILIANS Sixth Edition Joseph T. Collins TraVis W. TAGGart The Center for North American Herpetology THE CEN T ER FOR NOR T H AMERI ca N HERPE T OLOGY www.cnah.org Joseph T. Collins, Director The Center for North American Herpetology 1502 Medinah Circle Lawrence, Kansas 66047 (785) 393-4757 Single copies of this publication are available gratis from The Center for North American Herpetology, 1502 Medinah Circle, Lawrence, Kansas 66047 USA; within the United States and Canada, please send a self-addressed 7x10-inch manila envelope with sufficient U.S. first class postage affixed for four ounces. Individuals outside the United States and Canada should contact CNAH via email before requesting a copy. A list of previous editions of this title is printed on the inside back cover. THE CEN T ER FOR NOR T H AMERI ca N HERPE T OLOGY BO A RD OF DIRE ct ORS Joseph T. Collins Suzanne L. Collins Kansas Biological Survey The Center for The University of Kansas North American Herpetology 2021 Constant Avenue 1502 Medinah Circle Lawrence, Kansas 66047 Lawrence, Kansas 66047 Kelly J. Irwin James L. Knight Arkansas Game & Fish South Carolina Commission State Museum 915 East Sevier Street P. O. Box 100107 Benton, Arkansas 72015 Columbia, South Carolina 29202 Walter E. Meshaka, Jr. Robert Powell Section of Zoology Department of Biology State Museum of Pennsylvania Avila University 300 North Street 11901 Wornall Road Harrisburg, Pennsylvania 17120 Kansas City, Missouri 64145 Travis W. Taggart Sternberg Museum of Natural History Fort Hays State University 3000 Sternberg Drive Hays, Kansas 67601 Front cover images of an Eastern Collared Lizard (Crotaphytus collaris) and Cajun Chorus Frog (Pseudacris fouquettei) by Suzanne L. -
![Reptiles Squamata/Charinidae [ ] Lichanura Trivirgata Rosy Boa](https://docslib.b-cdn.net/cover/1134/reptiles-squamata-charinidae-lichanura-trivirgata-rosy-boa-2141134.webp)
Reptiles Squamata/Charinidae [ ] Lichanura Trivirgata Rosy Boa
National Park Service U.S. Department of the Interior Species Checklist for Mojave National Preserve (MOJA) This species list is a work in progress. It represents information currently in the NPSpecies data system and records are continually being added or updated by National Park Service staff. To report an error or make a suggestion, go to https://irma.nps.gov/npspecies/suggest. Scientific Name Common Name Reptiles Squamata/Charinidae [ ] Lichanura trivirgata rosy boa Squamata/Colubridae [ ] Arizona elegans glossy snake [ ] Chionactis occipitalis western shovel-nosed snake [ ] Coluber flagellum coachwhip [ ] Coluber taeniatus striped whipsnake [ ] Diadophis punctatus ring-necked snake [ ] Hypsiglena chlorophaea desert nightsnake [ ] Lampropeltis californiae California kingsnake [ ] Phyllorhynchus decurtatus spotted leaf-nosed snake [ ] Pituophis catenifer gopher snake [ ] Rhinocheilus lecontei long-nosed snake [ ] Salvadora hexalepis western patch-nosed snake [ ] Sonora semiannulata western groundsnake [ ] Tantilla hobartsmithi Smith's black-headed snake [ ] Trimorphodon biscutatus California lyresnake Squamata/Crotaphytidae [ ] Crotaphytus bicinctores Great Basin collared lizard [ ] Gambelia wislizenii long-nosed leopard lizard Squamata/Eublepharidae [ ] Coleonyx variegatus western banded gecko Squamata/Helodermatidae [ ] Heloderma suspectum gila monster Squamata/Iguanidae [ ] Dipsosaurus dorsalis desert iguana [ ] Sauromalus ater common chuckwalla [ ] Sceloporus occidentalis western fence lizard [ ] Sceloporus uniformis yellow-backed -

New Verified Nonindigenous Amphibians and Reptiles in Florida Through 2015, with a Summary of Over 152 Years of Introductions
WWW.IRCF.ORG/REPTILESANDAMPHIBIANSJOURNALTABLE OF CONTENTS IRCF REPTILES & IRCF AMPHIBIANS REPTILES • VOL &15, AMPHIBIANS NO 4 • DEC 2008 • 189 23(2):110–143 • AUG 2016 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS INTRODUCED SPECIES FEATURE ARTICLES . Chasing Bullsnakes (Pituophis catenifer sayi) in Wisconsin: New VerifiedOn the Road to Understanding the Nonindigenous Ecology and Conservation of the Midwest’s Giant Serpent ...................... Amphibians Joshua M. Kapfer 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: A Hypothetical Excursion ............................................................................................................................Robert W. Henderson 198 and ReptilesRESEARCH ARTICLES in Florida through 2015, with a . The Texas Horned Lizard in Central and Western Texas ....................... Emily Henry, Jason Brewer, Krista Mougey, and Gad Perry 204 Summary. The Knight Anole of(Anolis equestris over) in Florida 152 Years of Introductions .............................................Brian J. Camposano, Kenneth L. Krysko, Kevin M. Enge, Ellen M. Donlan, and Michael Granatosky 212 1 1 2 3 3 4 Kenneth L. KryskoCONSERVATION, Louis A. Somma ALERT, Dustin C. Smith , Christopher R. Gillette , Daniel Cueva , Joseph A. Wasilewski , 5 6 7 8 9 10 Kevin M. Enge. , Steve A. Johnson , Todd S. Campbell , Jake R. Edwards , Michael R. Rochford , Rhyan Tompkins , World’s Mammals11 in Crisis .............................................................................................................................................................12 -

Reproduction in the Baja California Collared Lizard, Crotaphytus Vestigium (Squamata: Crotaphytidae) Stephen R
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Occidental College Scholar Bulletin of the Southern California Academy of Sciences Volume 109 | Issue 3 Article 3 2010 Reproduction in the Baja California Collared Lizard, Crotaphytus vestigium (Squamata: Crotaphytidae) Stephen R. Goldberg Clark R. Mahrdt Follow this and additional works at: https://scholar.oxy.edu/scas Part of the Behavior and Ethology Commons, Biodiversity Commons, Other Ecology and Evolutionary Biology Commons, Population Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Goldberg, Stephen R. and Mahrdt, Clark R. (2010) "Reproduction in the Baja California Collared Lizard, Crotaphytus vestigium (Squamata: Crotaphytidae)," Bulletin of the Southern California Academy of Sciences: Vol. 109: Iss. 3. Available at: https://scholar.oxy.edu/scas/vol109/iss3/3 This Research Note is brought to you for free and open access by OxyScholar. It has been accepted for inclusion in Bulletin of the Southern California Academy of Sciences by an authorized editor of OxyScholar. For more information, please contact [email protected]. Goldberg and Mahrdt: Reproduction in the Baja California Collared Lizard Bull. Southern California Acad. Sci. 109(3), 2010, pp. 153–156 E Southern California Academy of Sciences, 2010 Research Note Reproduction in the Baja California Collared Lizard, Crotaphytus vestigium (Squamata: Crotaphytidae) Stephen R. Goldberg1 and Clark R. Mahrdt2 1Whittier College, Department of Biology, P.0. Box 634, Whittier, California 90608, USA, [email protected] 2Department of Herpetology, San Diego Natural History Museum, P.O. Box 121390, San Diego, California 92112-1390, USA, [email protected] Crotaphytus vestigium, a rock-dwelling species of the peninsular ranges of Baja California, occurs along the northern slope of the San Jacinto Mountains, Riverside County, California, south to the southern margin of the volcanic Magdalena Plain in Baja California Sur (McGuire 1996). -
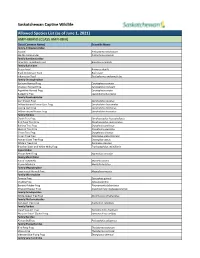
Captive Wildlife Allowed List
Saskatchewan Captive Wildlife Allowed Species List (as of June 1, 2021) AMPHIBIANS (CLASS AMPHIBIA) Class (Common Name) Scientific Name Family Ambystomatidae Axolotl Ambystoma mexicanum Marble Salamander Ambystoma opacum Family Bombinatoridae Oriental Fire-Bellied Toad Bombina orientalis Family Bufonidae Green Toad Anaxyrus debilis Black Indonesian Toad Bufo asper Indonesian Toad Duttaphrynus melanostictus Family Ceratophryidae Surinam Horned Frog Ceratophrys cornuta Chacoan Horned Frog Ceratophrys cranwelli Argentine Horned Frog Ceratophrys ornata Budgett’s Frog Lepidobatrachus laevis Family Dendrobatidae Dart Poison Frog Dendrobates auratus Yellow-banded Poison Dart Frog Dendrobates leucomelas Dyeing Dart Frog Dendrobates tinctorius Yellow-striped Poison Frog Dendrobates truncatus Family Hylidae Clown Tree Frog Dendropsophus leucophyllatus Bird Poop Tree Frog Dendropsophus marmoratus Barking Tree Frog Dryophytes gratiosus Squirrel Tree Frog Dryophytes squirellus Green Tree Frog Dryophytes cinereus Cuban Tree Frog Osteopilus septentrionalis Haitian Giant Tree Frog Osteopilus vastus White’s Tree Frog Ranoidea caerulea Brazilian Black and White Milky Frog Trachycephalus resinifictrix Hyperoliidae African Reed Frog Hyperolius concolor Family Mantellidae Baron’s Mantella Mantella baroni Brown Mantella Mantella betsileo Family Megophryidae Long-nosed Horned Frog Megophrys nasuta Family Microhylidae Tomato Frog Dyscophus guineti Chubby Frog Kaloula pulchra Banded Rubber Frog Phrynomantis bifasciatus Emerald Hopper Frog Scaphiophryne madagascariensis -

Sexual Dichromatism and Differential Conspicuousness in Two Populations of the Common Collared Lizard (Crotaphytus Collaris) from Utah and New Mexico, USA
Blackwell Science, LtdOxford, UKBIJBiological Journal of the Linnean Society0024-4066The Linnean Society of London, 2002 77 Original Article J. M. MACEDONIA ET AL.COLOUR VARIATION IN CROTAPHYTUS COLLARIS Biological Journal of the Linnean Society, 2002, 77, 67–85. With 8 figures Sexual dichromatism and differential conspicuousness in two populations of the common collared lizard (Crotaphytus collaris) from Utah and New Mexico, USA JOSEPH M. MACEDONIA1*, YONI BRANDT2,3 and DAVID L. CLARK4 1Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville, TN 37996 USA 2Department of Biology and 3Center for the Integrative Study of Animal Behaviour, Indiana University, Bloomington, Indiana 47405 USA 4Department of Biology, Alma College, Alma, MI 48801 USA Received 28 November 2001; accepted for publication 7 May 2002 The common collared lizard (Crotaphytus collaris) exhibits considerable geographical colour variation, particularly among males. Populations of this diurnal saxicolous iguanian inhabit patches of rocky habitat throughout the spe- cies’ broad distribution in North America and are anticipated to experience local differences in selective pressures that influence colouration. Specifically, while social interactions might favour conspicuous colouration, crypsis may be advantageous in interactions with visually orienting predator and prey species. To address the local relationship between lizard and substrate colouration we compared the reflectance spectra of two geographically distant and phe- notypically divergent populations of collared lizards with the rocky substrates they inhabit. Our northern study pop- ulation (C. c. auriceps in eastern Utah) occurs on red rocks, where males exhibit boldly coloured turquoise bodies and bright yellow heads. In contrast, our southern study population (C. c. fuscus in southern New Mexico) lives on grey and tan rocks, and males in this location exhibit subdued brown and tan dorsal colours.