Intra-Annual Variations of Arsenic Totals and Species in Tropical Estuary Surface Sediments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(DDRC), East Godavari District, Andhra Pradesh Implementing Agency Uma Educational
District Disability Rehabilitation Centre (DDRC), East Godavari District, Andhra Pradesh Implementing Agency Uma Educational and Technical Society , Kakinada List of Beneficiaries Type Ge of Required Date Regd. No. Name of the PWD Guardian Name nd Age Caste D.No Village Mandal District Contact No. Remarks Disa Needs er bilit S.NO y 1 04-01-2016 DDRC/849/2016 Kopparthi Praveen Kumar Venkateswara Rao M 31 OC 1-22/8/2, Sriramnagar Kakinada East Godavari 8121912976 OH Tri Cycle Penddig 2 04-01-2016 DDRC/850/2016 Kadite Enosh Babu Yesubabu M 22 SC Mallam Pithapuram East Godavari 9963412280 OH Wheel Chair Penddig 3 04-02-2016 DDRC/851/2016 Yekula Veera Mallikajunudu Styanarayana M 29 BC 2-32, Veeravaram Kadiyam East Godavari 9573211202 OH Tri Cycle Penddig AFO,Knee Cap 4 04-02-2016 DDRC/852/2016 Vasamsetti Satya Kumari Srinivasu F 29 BC 12-393 Polamuru Anaparthi East Godavari 9949386191 OH KAFO( R) Changed 5 04-02-2016 DDRC/853/2016 Nagavarapu Suryanarayana Suranna M 55 OC 1-130 Damireddypalli Kadiyam East Godavari 9966139266 OH BK Limb(L) J-Foot ,Belt Changed 6 04-02-2016 DDRC/854/2016 Singana Appa Rao Venkanna M 72 OC 3-120 Madhavarayudupalem Kadiyam East Godavari HH Hearing Aid Penddig 7 04-02-2016 DDRC/855/2016 Dangudubiyyam Nagamani Subba Rao F 60 OC 6-72, Jagatahavari Veedhi Kadiyam East Godavari 9618484827 OH BK Limb( R) Taken Mesurement 8 04-02-2016 DDRC/856/2016 Busi Narasimha Rao Chandra Rao M 60 BC 11-0130 Challapalli Uppalaguptam East Godavari 9542082062 OH BK Limb( R) Taken Mesurement 9 04-02-2016 DDRC/857/2016 Ulisi Chantamma -

East Godavari District Annual Report
OM SRI SAIRAM East Godavari District Annual Report st st from 1 April 2018 - 31 March 2019 Contents FOREWORD FROM THE DISTRICT PRESIDENT ............................................................... SRI SATHYA SAI SEVA ORGANISATIONS – AN INTRODUCTION ......................................... WINGS OF THE ORGANISATIONS .............................................................................................. ADMINISTRATION OF THE ORGANISATION ............................................................................... THE 9 POINT CODE OF CONDUCT AND 10 PRINCIPLES ...................................................................... SRI SATHYA SAI SEVA ORGANISATIONS, [EAST GODAWARI District] ................................. BRIEF HISTORY .................................................................................................................................... DIVINE VISIT .............................................................................................................................. OVERVIEW ................................................................................................................................ SAI CENTRES ....................................................................................................................................... ACTIVITIES ................................................................................................................................ OFFICE BEARERS ............................................................................................................................... -

Memory and the Recovery of Identity: Living Histories and The
9 Memory and the Recovery of Identity Living Histories and the Kalavantulu of Coastal Andhra Pradesh DAVESH SONEJI Peddapuram is a town located in the east Godavari district of Andhra Pradesh, about 20 km from the city of Kakinada. It is famous for its celebration of the annual festival (jatra) of the local goddess Maridamma2 in the month ofJyaishtha, and infamous for its rows of brothels. Today, the town of Peddapuram is known for the exceptionally high numbers of pros- titutes who inhabit its streets. Many of them are kalavantulu. These women work in the brothels of towns like Peddapuram as the result of a complex social, political, aesthetic and cultural restructuring that began in the nine- teenth century. One of the senior-most kalavantulu living in Peddapuram is Jakkula Radha. Today Radha sells bidis (tendu-leaf cigarettes), candy, and other confectionary at a small stall outside her home. For about an hour after I met her, Radha refused to discuss her kalavantula identity. Instead, she talked about the fact that she has converted to Christianity, because the local mission pays her Rs 60 (about $2) per month for maintaining a Christian lifestyle. 3 When she finally began to speak openly about her past, she insisted on continuing our conversation elsewhere. We helped her into our Ambassador car and started to back out of the lane on which her house "V 285 284 Performing Pasts Memory and the Recovery of Identity was located. Sure enough, four young men rushed out from nearby houses, and provide us with insights that cannot be found elsewhere. -

East Godavari District
East Godavari district S.No. Name of the Health care facility 1. Veterinary Biological Reasearch Institute (VBRI),Sy.No. 592, Ragampeta Road, Samalkot, East Godavari District. 2. South Central Railway Health Unit, Near Railway Station, Tuni, East Godavari District. 3. South Central Railway Hospital,Near Railway Station,Samalkot,East Godavari District. 4. Employees State Insurance Hospital, Sreeram nagar, Rajamahendravaram, East Godavari District. 5. District Hospital, Lala Cheruvu Road, Near Central Prison, Rajamahendravaram, East Godavari District. 6. Area Hospital Amalapuram. KNF Road, Near Black Bridge East Godavari District 7. Area Hospital, Tuni, East Godavari District. 8. Area Hospital, Medical Superintendent, Ramachandrapuram,East Godavari District. 9. Community Health Center, Anaparthy, East Godavari District. 10. Community Health Centre (CHC), Pandavula Metta,Peddapuram,East Godavari District. 11. Community Health Center, Kadiy am, East Godavari District. 12. Community Health Centre (CHC), Alamuru Road, Mandapeta, East Godavari District 13. Community Health Center, Allavaram, East Godavari District. 14. Community Health Centre (CHC), P.Gannavaram,East Godavari District 15. Community Health Centre (CHC), Pension Line,Samalkot, East Godavari District. 16. G.S.L. Educational Society,G.S.L Medical College & General Hospital, NH -16, Lakhsmipuram, Rajamahendravaram, East Godavari District. 17. Konaseema Institute of Medical Sciences and Research Foundation & Kims General Hospitals(Mother theressa Educational society), NH-214, Chaitanya Health city, Kamanagaruvu (V), Amalapuram(M),East Godavari District 18. Lenora Institute of Dental Science, (Lenora Dental Hospital), Near Petro l Pump, NH -16, Rajanagaram (V), Rajamahendravaram, East Godavari District. 19. Raju Neuro Multispeciality Hospital Pvt Ltd., D.No. 76 -4-7, Gandhipuram -2, Rajamahendravaram, East Godavari District 20. Swatantra Hospitals (Multispecialities) Private Limited, D .no:19 -5-21/6, Near Kambala Park, Rajamahendravaram,East Godavari District 21. -

Census Handbook, East Godavari
1951 CENSUS HANDBOOK EAST GODAVARI DISTRICT PRINTED BY THE SUPERIN':CENDENT GOVERNMENT PRESS MADRAS 1953 CONTENTS PAGE PAGlI PARi' I---tJtmI. 1 PREFAOE v Section (ii). 2 Introductory note about the district with annexures .. 1 8 Rural Stati8tic8-(Information regarding area, n1lID.ber of occupied hou~es, literacy, distribution of popula STATISTICS. tion by livelihood classes, cultivated area, small-scale industrial ~t8btishments and incidence pf leprosy in villag!'s) with appendix giving a list of villages with PART I. a population exceeding 5;,000 but treated as rural •• 91 Section (i). (iii). 3 'A ' General Population Tables- • sekon, I 9 Urban Statistics-:-(Inform8.tion regarding area, number A-I-Area, Hou'Ses and Population 14 of occupied hOUses, lite1'8cy, distribution of popula tion by liH,lihood classes, ,small-scale industrial A-II-Variation in Population during fifty years 15 establishments and incidence. of leprosy in each ward of each ()enBW town and city) .. 239 A-III-Towns and VillageR classified by Population 17 A-IV-Cities and Towns classified by Population with PART II. variatioDs since 1901 19 10 'b' Household ~nd Age (Sample) Tables- A-V-Towns arranged talukwise with Population by C-I-Household (size) 272 Livelihood Classes 22. C-II-Livelihood Classes by Age.groups .. 274 4 • E' Summary Figures by taluks 24 C-IV-Age and Literacy 276 5 'B ' Economic Tables 11 • D' Social and Gultural Tables- B-I-Livelihood Classe~ and Sub-classes 27 D-I-Languages- B-II-Secondary means of Livelihood 32 (i) Mother-tongue 280 B-III-Employers, Employees and Independent Workers (ii) Bi-lingualism 283 in Industries and Services by Divisions and Subdivisions 36 D-II-Religion 290 D-III-Scheduled Castes and Scheduled Tribes. -

Disaster Management E. Godavari District
Introduction The East Godavari District is located in the North Coastal part of the state of Andhra Pradesh. The District boundaries are Visakhapatnam, West Godavari, Khammam Districts and Bay of Bengal.The District is known as rice bowl of Andhra Pradesh with lush paddy fields and coconut groves. It is also known as another Kerala. East Godavari, it is the Rice Granary of Andhra Pradesh, beckons tourists to have a glimpse of its rich cultural heritage. Where the lush paddy fields swaying in the breeze appear to dance in a celebration to life.The Headquarters of the District is located at Kakinada.The District is a residuary portion of the old Godavari District after West Godavari District was separated in 1925. As the name of the district conveys, East Godavari District is closely associated with the river Godavari, occupying a major portion of the delta area. East Godavari district is having the area of 10,807 Sq Kms with 7 Revenue divisions, 64 Revenue mandals and 1012 Grama panchayats with a population of 51,51,549 as per 2011 provisional census figures. The Headquarters of the District is located at Kakinada. East Godavari District lies North - East Coast of Andhra Pradesh and bounded on the North by Visakhapatnam District and the State of Orissa, on the East and the South by the Bay of Bengal and on the West by Khammam District of Telangana State and West Godavari Districts. Area of the District is 12,805 Sq.Kms including newly added Yetapaka Division. The District is located between Northern latitudes of 16o 30' and 18o 20' and between the Eastern longitudes of 81o 30' and 82o 30'. -

Pre-Feasibility Report
PRE-FEASIBILITY REPORT FOR PROPOSED AMMONIA & NPK FERTILISER COMPLEX At N. Kothapalli & Gachakayalapora Villages, Uppalaguptam & Katrenikona Mandals East Godavari District, Andhra Pradesh, INDIA Project Proponent: M/s Konaseema Fertilizers & Chemicals Limited 6-2-913/914,2nd Floor, Progressive Towers Khairatabad, Hyderabad-500004, India Mail : [email protected], [email protected] CIN No. U10100AP1996PLC024035 Environment Consultant: Bhagavathi Ana Labs Pvt. Limited (A Bureau Veritas Group Company) 7-2-C-14, Industrial Estate, Sanathnagar, Hyderabad 500018 August 2017 Page 1 of 34 CHAPTER-I EXECUTIVE SUMMARY For manufacture of Ammonia, which is essential for production Urea, DAP, NPK fertilizers, the feed stock is natural gas. Hindustan LNG company has taken steps to install a project for importation of Liquified Natural Gas (LNG) and re-gasify the LNG to convert it into gas for supply to various consumers. The HLNG Project will be coming up near a port in East Godavari District, which is being set up by Andhra Pradesh State Government. Fertilizer units are the major consumers of natural gas. Konaseema Fertilizers & Chemicals Limited (KFCL) proposes to take advantage of availability of natural gas as feed stock from HLNG and accordingly is taking steps to establish a large fertilizer complex in proximity to the HLNG project. This location will enable KFCL for obtaining natural gas at a cheaper price, as substantial charges involved for transportation of the gas to distant locations can be avoided. The other major advantage for locating the fertilizer complex near a port is importation of large quantities of raw materials like sulphur, potash and MOP required for manufacture of Sulphuric Acid and Phosphoric Acid and phosphatic fertilizers. -

Answered On:11.12.2000 Expansion of Telephone Exchanges Srinivasulu Kalava
GOVERNMENT OF INDIA COMMUNICATIONS LOK SABHA UNSTARRED QUESTION NO:3419 ANSWERED ON:11.12.2000 EXPANSION OF TELEPHONE EXCHANGES SRINIVASULU KALAVA Will the Minister of COMMUNICATIONS be pleased to state: (a) whether the Government propose to expand the capacity of existing telephone exchanges and develop the infrastructural facility of telephone of East and West Godavari district; (b) if so, the details thereof, location and district-wise; and (c) the steps taken by the Government in this regard? Answer THE MINISTER OF STATE IN THE MINISTRY OF COMMUNICATIONS (SHRI TAPAN SIKDAR) (a) Yes, Sir. (b) & (c): Details of locations of existing telephone exchanges, district-wise, planned for expansion during 2000-2001 are given at Annexure. All exchanges except the following are working on reliable media in East and West Godavari District: S.No. District Location 1. East Godavari 1. Atchutapuram 2. Kesavaram 3. Vanapalli 4. Devipatnam 5. Maredumilli 2. West Godavari 1. P.V.Gudem 2. Gunnepalli 3. Ch.Chintalapudi It is planned to provide reliable media for above exchanges during 2000-2001. Aerial and OFC cables have been allotted and work to provide reliable media is in progress. ANNEXURE Details of expansions planned for 2000-2001 A. East Godavari district S.No. Location Capacity addition in lines 1. Amallavaram 184 2. A.V.Nagaram 184 3. 2Addatheegala 184 4. Alamuru 856 5. Allavaram 424 6. Amalapuram 1000 7 Ambajipeta (AMP) 500 8. Anaparthi 500 9. Anathavaram 416 10. Angara 704 11. Annavaram 400 12. Antharvedi 432 13. Anuru 184 14. Atreyapuram 304 15. Bikkavole (RMM) 372 16. C.S.K.Pudi 128 17. -
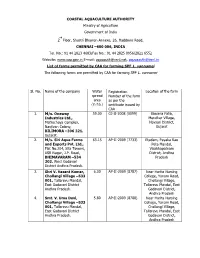
Total Applications Received for Granting Permission to Undertake SPF L
COASTAL AQUACULTURE AUTHORITY Ministry of Agriculture Government of India nd 2 Floor, Shastri Bhawan Annexe, 26, Haddows Road, CHENNAI –600 006, INDIA Tel. No.: 91 44 2823 4683/Fax No.: 91 44 2825 0956/2821 6552 Website: www.caa.gov.in E-mail: [email protected], [email protected] List of farms permitted by CAA for farming SPF L. vannamei The following farms are permitted by CAA for farming SPF L. vannamei Sl. No. Name of the company Water Registration Location of the farm spread Number of the farm area as per the (in ha.) certificate issued by CAA 1. M/s. Onaway 59.00 GJ-II-2008 (0099) Bawaria Falia, Industries Ltd., Mendher Village, Matruchaya Complex, Navsari District, Navjivan Colony, Gujarat BILIMORA –396 321. Gujarat. 2. M/s. Siri Aqua Farms 63.15 AP-II-2009 (7733) Etadam, Payaka Rao and Exports Pvt. Ltd., Peta Mandal, Flat No.204, Sita Towers, Visakhapatnam ASR Nagar, J.P. Road, District, Andhra BHIMAVARAM –534 Pradesh 202. West Godavari District Andhra Pradesh. 3. Shri V. Vasant Kumar, 6.00 AP-II-2009 (8787) Near Harita Nursing Chollangi Village –533 College, Yanam Road, 001, Tallarevu Mandal, Chollangi Village, East Godavari District Tallarevu Mandal, East Andhra Pradesh. Godavari District, Andhra Pradesh 4. Smt. V. Uma Devi, 5.80 AP-II-2009 (8788) Near Harita Nursing Chollangi Village –533 College, Yanam Road, 001, Tallarevu Mandal, Chollangi Village, East Godavari District Tallarevu Mandal, East Andhra Pradesh. Godavari District, Andhra Pradesh 5. M/s. Prathyusha 21.00 AP-II-2009 (7749) Ravivaripalem Village, Global Trade Pvt. Ltd., Tangutur Mandal, Ravivaripalem Village Prakasam District Prakasam District. -
1. Salient Features of East Godavari District
District Survey Report - 2018 1. Salient Features of East Godavari District East Godavari District which is one of the largest and the most populous district in the state of Andhra Pradesh, was successively ruled by Mauryas, Sathavahanas, Vishnu Kundins, Eastern Chalukyas, Cholas, Kakatiyas, Musunuri Chieftains, Kondaveeti Reddy Kings, Gajapathis, Kutubshahis and then by the British. It was under the Raj. On April 15, 1925, East Godavari District was formed as per G.O.No.502. East Godavari is the richest district of the state, in terms of GDP. It is both the cultural and tourist hub of the state. East Godavari is a district in Coastal Andhra region of Andhra Pradesh, India. Its district headquarters is at Kakinada. As of Census 2011, it became the most populous district of the state. Rajahmundry and Kakinada are the two large cities in the Godavari districts in terms of population. The district of Rajahmundry (present name Rajamahendravaram) was reorganised in 1859 into two districts of Godavari and Krishna. Godavari District was further bifurcated into East and West Godavari districts in 1925. After Nov 1956, Andhra Pradesh was formed by combining parts of Naizam, Ceded and Circars. In 1959, the Bhadrachalam revenue division, consisting of Bhadrachalam and Naguru Taluqs (2 Taluqas in 1959 but later subdivided into Wajedu, Venkatapruram, Charla, Dummugudem, Bhadrachalam, Nellipaka, Chinturu, Kunavaram, and Vara Rama Chandra Puram mandals) of East Godavari district were merged into the Khammam district. After June 2014's reorganisation and division of Andhra Pradesh, the Mandals of Bhadrachalam (with the exception of Bhadrachalam Temple), Nellipaka, Chinturu, Kunavaram and Vara Rama Chandra Puram were re-added to East Godavari District. -

Unfinished Gestures South Asia Across the Disciplines
unfinished gestures South Asia Across the Disciplines A series edited by Dipesh Chakrabarty, Sheldon Pollock, and Sanjay Subrahmanyam Funded by a grant from the Andrew W. Mellon Foundation and jointly pub- lished by the University of California Press, the University of Chicago Press, and Columbia University Press. The Powerful Ephemeral: Everyday Healing in an Ambiguously Islamic Place by Carla Bellamy (California) Extreme Poetry: The South Asian Movement of Simultaneous Narration by Yigal Bronner (Columbia) Secularizing Islamists? Jama‘at-e-Islami and Jama‘at-ud Da‘wa Pakistan by Humeira Iqtidar (Chicago) The Social Space of Language: Vernacular Culture in British Colonial Punjab by Farina Mir (California) Unifying Hinduism: Philosophy and Identity in Indian Intellectual History by Andrew J. Nicholson (Columbia) Islam Translated: Literature, Conversion, and the Arabic Cosmopolis of South and Southeast Asia by Ronit Ricci (Chicago) South Asia across the Disciplines is a series devoted to publishing fi rst books across a wide range of South Asian studies, including art, history, philology or textual studies, philosophy, religion, and the interpretive social sciences. Series authors all share the goal of opening up new archives and suggesting new methods and approaches, while demonstrating that South Asian schol- arship can be at once deep in expertise and broad in appeal. unfinished gestures Devada¯sı¯s, Memory, and Modernity in South India davesh soneji the university of chicago press chicago and london Davesh Soneji is associate professor of South Asian religions at McGill University. He is coeditor of Performing Pasts: Reinventing the Arts in Modern South India and editor of Bharatanatyam: A Reader. -

Assessment of Ecological Settings and Biodiversity
Assessment of Ecological Settings and Biodiversity Values of Papikonda National Park and Indira Sagar (Polavaram) Multipurpose Project Impact Zone in Andhra Pradesh for Development of Mitigatory Measures FINAL REPORT September 2014 Citation: Sivakumar, K., J.A. Johnson, N. Gokulakkannan, Paromita Ray, Gitanjali Katlam, Priyamvada Bagaria, 2014. Assessment of Ecological Settings and Biodiversity Values of Papikonda National Park and Indira Sagar (Polavaram) Multipurpose Project Impact Zone in Andhra Pradesh for Development of Mitigatory Measures. Wildlife Institute of India, Dehradun. Assessment of Ecological Settings and Biodiversity Values of Papikonda National Park and Indira Sagar (Polavaram) Multipurpose Project Impact Zone in Andhra Pradesh for Development of Mitigatory Measures FINAL REPORT PRINCIPAL INVESTIGATOR Dr. K. Sivakumar CO-INVESTIGATOR Dr. J.A. Johnson RESEARCHERS N. Gokulakkannan Paromita Ray Gitanjali Katlam Priyamvada Bagaria September 2014 Ecological Settings and Biodiversity Values of Indira Sagar (Polavaram) Multipurpose Project impact zone TABLE OF CONTENTS Chapter Topic Page No. 1 Summary 1 2 Introduction 15 3 Objectives of the Study 22 4 Geographical Scope of the Study 23 5 General Methodology 27 6 Status and Distribution of Fauna and Flora in the 33 Submergence Zone Inside Papikonda National Park 7 Status and Distribution of Fish Diversity in the Impact 64 Zone of the Project in the River Godavari 8 Riparian Vegetation Index 75 9 Potential Impact of the Project 95 10 Mitigation and Recommendations for the Polavaram 107 Project 11 Environmental Flows 116 References 149 Annexures 157 i Ecological Settings and Biodiversity Values of Indira Sagar (Polavaram) Multipurpose Project impact zone Chapter 1 SUMMARY The Indira Sagar (Polavaram Project) Multipurpose Project envisages the construction of a zoned earth-cum-rock fill dam with an impervious core across the Godavari River in Polavaram.