Large Complex Burrows of Terrestrial Invertebrates: Neoichnology of Pandinus Imperator (Scorpiones: Scorpionidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Target-Specificity in Scorpions
Toxins 2017, 9, 312, doi: 10.3390/toxins9100312 S1 of S3 Supplementary Materials: Target‐specificity in scorpions; Comparing lethality of scorpion venoms across arthropods and vertebrates Arie van der Meijden, Bjørn Koch, Tom van der Valk, Leidy J. Vargas‐Muñoz and Sebastian Estrada‐Gómez Table S1. Table with GenBank CO1 accession numbers. Voucher numbers refer to the specimens in the collection of CIBIO/InBio at the University of Porto. Species Voucher Accession number Androctonus australis Sc904 gi379647585 Leiurus quinquestriatus Sc1062 gi379647601 Buthus ibericus Sc112 gi290918312 Grosphus flavopiceus Sc1085 gi379647593 Centruroides gracilis gi71743793 Heterometrus laoticus Sc1084 gi555299473 Pandinus imperator Sc1050 gi379647587 Hadrurus arizonensis Sc1042 gi379647597 Iurus kraepelini Sc866 gi379647589 Table S2. Mean dry venom compound per individual. Several milkings were made per (sub)adult specimen in some cases. Species n Dry venom (mg) mg/milking Androctonus australis 21 23.5 1.12 Leiurus quinquestriatus 51 25.1 0.49 Buthus ibericus 47 41.6 0.89 Centruroides gracilis 42 22.5 0.54 Babycurus jacksoni 38 53.9 1.42 Grosphus grandidieri 19 103.9 5.47 Hadrurus arizonensis 9 75.3 8.37 Iurus sp.* 12 35.2 2.93 Heterometrus laoticus 21 119 5.67 Pandinus imperator 15 72.7 4.85 *2 I. dufoureius, 6 I. kraepelini, 4 I. sp. Toxins 2017, 9, 312, doi: 10.3390/toxins9100312 S2 of S3 Table S3. Toxicological analysis of the venom of Grosphus grandidieri. “Toxic” means that the mice showed symptoms such as: pain, piloerection, excitability, salivation, lacrimation, dyspnea, diarrhea, temporary paralysis, but recovered within 20 h. “Lethal” means that the mice showed some or all the symptoms of intoxication and died within 20 h after injection. -

The Scorpion Fauna of Mona Island, Puerto Rico (Scorpiones: Buthidae, Scorpionidae)
The Scorpion Fauna of Mona Island, Puerto Rico (Scorpiones: Buthidae, Scorpionidae) Rolando Teruel, Mel J. Rivera & Alejandro J. Sánchez August 2017 – No. 250 Euscorpius Occasional Publications in Scorpiology EDITOR: Victor Fet, Marshall University, ‘[email protected]’ ASSOCIATE EDITOR: Michael E. Soleglad, ‘[email protected]’ Euscorpius is the first research publication completely devoted to scorpions (Arachnida: Scorpiones). Euscorpius takes advantage of the rapidly evolving medium of quick online publication, at the same time maintaining high research standards for the burgeoning field of scorpion science (scorpiology). Euscorpius is an expedient and viable medium for the publication of serious papers in scorpiology, including (but not limited to): systematics, evolution, ecology, biogeography, and general biology of scorpions. Review papers, descriptions of new taxa, faunistic surveys, lists of museum collections, and book reviews are welcome. Derivatio Nominis The name Euscorpius Thorell, 1876 refers to the most common genus of scorpions in the Mediterranean region and southern Europe (family Euscorpiidae). Euscorpius is located at: http://www.science.marshall.edu/fet/Euscorpius (Marshall University, Huntington, West Virginia 25755-2510, USA) ICZN COMPLIANCE OF ELECTRONIC PUBLICATIONS: Electronic (“e-only”) publications are fully compliant with ICZN (International Code of Zoological Nomenclature) (i.e. for the purposes of new names and new nomenclatural acts) when properly archived and registered. All Euscorpius issues starting from No. 156 (2013) are archived in two electronic archives: • Biotaxa, http://biotaxa.org/Euscorpius (ICZN-approved and ZooBank-enabled) • Marshall Digital Scholar, http://mds.marshall.edu/euscorpius/. (This website also archives all Euscorpius issues previously published on CD-ROMs.) Between 2000 and 2013, ICZN did not accept online texts as "published work" (Article 9.8). -

Scorpion Venoms in Gastric Cancer (Review)
ONCOLOGY LETTERS 12: 3683-3686, 2016 Scorpion venoms in gastric cancer (Review) XIAO-YING ZHANG1 and PEI-YING ZHANG2 1Nanjing University of Chinese Medicine, Information Institute, Nanjing; 2Department of Cardiology, Xuzhou Central Hospital, The Affiliated Xuzhou Hospital of Medical College of Southeast University, Xuzhou, Jiangsu 221009, P.R. China Received April 27, 2016; Accepted September 1, 2016 DOI: 10.3892/ol.2016.5134 Abstract. Venom secretions from snakes, scorpions, spiders anemones (1). The venoms could be secreted in teeth, stingers, and bees, have been widely applied in traditional medicine claws or even the skins of these animals, to paralyze and kill and current biopharmaceutical research. Possession of anti- the prey or to protect themselves from predation and other cancer potential is another novel discovery for animal venoms dangers. The signs and symptoms after exposure to venoms and toxins. An increasing number of studies have shown vary from mild allergic reactions including itch, pain, swelling the anticancer effects of venoms and toxins of snakes, and to respiratory arrest, paralysis, necrosis or even death (2). The scorpions in vitro and in vivo, which were achieved mainly utilization of animal venoms in folk medicine has been docu- through the inhibition of cancer growth, arrest of cell cycle, mented for a long time in some countries, such as China, India induction of apoptosis and suppression of cancer metastasis. and the Middle East. For example, Chan Su, the dried toad However, more evidence is needed to support this concept and venom from skin glands, first recorded in traditional Chinese the mechanisms of anticancer actions are not clearly under- medicine more than 1,000 years ago, has been long used as a stood. -

Arachnides 76
Arachnides, 2015, n°76 ARACHNIDES BULLETIN DE TERRARIOPHILIE ET DE RECHERCHES DE L’A.P.C.I. (Association Pour la Connaissance des Invertébrés) 76 2015 0 Arachnides, 2015, n°76 LES PREDATEURS DES SCORPIONS (ARACHNIDA : SCORPIONES) G. DUPRE Dans leur revue sur les prédateurs de scorpions, Polis, Sissom & Mac Cormick (1981) relèvent 150 espèces dont essentiellement des espèces adaptées au comportement nocturne de leur proie (chouettes, rongeurs, carnivores nocturnes) mais également des espèces diurnes (lézards, rongeurs, carnivores....) qui débusquent les scorpions sous les pierres ou dans leurs terriers. Dans une précédente note (Dupré, 2008) nous avions effectué un relevé afin d'actualiser cette étude de 1981. Sept ans après, de nouvelles données sont présentées dans cette synthèse. Voici un nouveau relevé des espèces prédatrices. Nous ne faisons pas mention des scorpions qui feront l'objet d'un futur article traité avec le cannibalisme. Explication des tableaux: La première colonne correspond aux prédateurs, la seconde aux régions concernées et la troisième aux références. Dans la mesure du possible, les noms scientifiques ont été rectifiés en fonction des synonymies ou des nouvelles combinaisons appliquées depuis les dates de publication d'origine. ARTHROPODA ARACHNIDA SOLIFUGAE Solifugae Afrique du Nord Millot & Vachon, 1949; Punzo, 1998; Cloudsley-Thompson, 1977 Eremobates sp. USA Bradley, 1983 ARACHNIDA ARANEAE Acanthoscurria atrox Brésil Lourenço, 1981 Aphonopelma sp. et autres Amérique centrale Mazzotti, 1964 Teraphosidae Phormictopus auratus Cuba Teruel & De Armas, 2012 Brachypelma vagans Mexique Dor et al., 2011 Epicadus heterogaster Brésil Lourenço et al. 2006 Latrodectus sp. USA Baerg, 1961 L. hesperus USA Polis et al., 1981 L. mactans Cuba Teruel, 1996; Teruel & De Armas, 2012 L. -

Reanalysis of the Genus Scorpio Linnaeus 1758 in Sub-Saharan Africa and Description of One New Species from Cameroon
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomologische Mitteilungen aus dem Zoologischen Museum Hamburg Jahr/Year: 2011 Band/Volume: 15 Autor(en)/Author(s): Lourenco Wilson R. Artikel/Article: Reanalysis of the genus Scorpio Linnaeus 1758 in sub-Saharan Africa and description of one new species from Cameroon (Scorpiones, Scorpionidae) 99-113 ©Zoologisches Museum Hamburg, www.zobodat.at Entomol. Mitt. zool. Mus. Hamburg15(181): 99-113Hamburg, 15. November 2009 ISSN 0044-5223 Reanalysis of the genus Scorpio Linnaeus 1758 in sub-Saharan Africa and description of one new species from Cameroon (Scorpiones, Scorpionidae) W ilson R. Lourenço (with 32 figures) Abstract For almost a century, Scorpio maurus L., 1758 (Scorpiones, Scorpionidae) has been considered to be no more than a widespread and presumably highly polymorphic species. Past classifications by Birula and Vachon have restricted the status of different populations to subspecific level. In the present paper, and in the light of new evidence, several African populations are now raised to the rank of species. One of these, Scorpio occidentalis Werner, 1936, is redescribed and a neotype proposed to stabilise the taxonomy of the group. A new species is also described from the savannah areas of Cameroon. This is the second to be recorded from regions outside the Sahara desert zone. Keywords: Scorpiones, Scorpionidae, Scorpio, new rank, new species, Africa, Cameroon. Introduction The genus Scorpio was created by Linnaeus in 1758 (in part), and has Scorpio maurus Linnaeus, 1758 as its type species, defined by subsequent designation (Karsch 1879; see also Fet 2000). -

Full-Text (PDF)
Vol. 6(1), pp. 21-28, January 2014 DOI: 10.5897/JMA2013.0286 ISSN 2141-2308 ©2014 Academic Journals Journal of Microbiology and Antimicrobials http://www.academicjournals.org/JMA Full Length Research Paper Investigation of the antimicrobial and hemolytic activity of venom of some Egyptian scorpion Wesam Salama* and Naglaa Geasa Zoology Department, Faculty of Science, Tanta University, Egypt. Accepted 16 January, 2014 Leuirus quinquestriatus, Androctonus amoreuxi and Androctonus australis are venomous scorpion in Egypt. Their venom is complex mixture of salts, neurotoxins, peptides and proteins which has therapeutic applications, and can rapidly kill a broad range of microbes. Estimations of total proteins of the venom indicated that L. quinquestriatus had the highest value than the others (10.64 ± 0.04). Hemolytic activity of human erythrocytes was detected and showed that all concentrations (20, 8 and 5 mg/ml) of tested scorpion species crude venom have hemolytic activity against human erythrocytes. The present study was conducted to evaluate the antimicrobial activity of the three Egyptian scorpion’s venom against four Gram-positive and negative bacterial strains (Bacillus cereus, Bacillus subtillis, Citrobacter freundi and Klibsella pneumonia) in addition to one fungus species Candida albicans. The results show that L. quinquestriatus venom has a significant antibacterial effect against B. subtillis and C. freundi. In contrast, A. amoreuxi and A. australis venoms do not have a noticeable effect on tested microbes. So, the aim of the present study was to investigate the antimicrobial activity of crude venom against Gram-negative, as well as Gram-positive bacteria, fungi and to evaluate the hemolytic activity of the three investigated species on human erythrocytes. -

Emperor Scorpion Class: Arachnida
Pandinus imperator Emperor Scorpion Class: Arachnida. Order: Scorpiones. Family: Scorpionidae. Other names: Imperial Scorpion Physical Description: One of the largest of scorpions in the world, the emperor scorpion has a dark body ranging from dark blue/green through brown to black and reaches lengths up to 8”. The large pincers are blackish-red and have a granular texture. The front part of the body is made up of four sections, each with a pair of legs. Behind the fourth pair of legs are comb-like structures known as pectines, which are sensory appendages that brush the substrate as the scorpion walks. The pectines are paired and can are used to distinguish sexes, as the ventral appendages are longer in males than females. The tail is long and curves back over the body ending with a large receptacle containing the venom glands and tipped with the sharp, curved stinger. Sensory hairs cover the pincers and tail, enabling the scorpion to detect prey through air and ground vibrations. Diet in the Wild: Insects, arachnids, mice and small lizards Diet at the Zoo: Crickets Habitat & Range: The emperor scorpion is found in western Africa and Congo areas, typically in hot and humid forests. They reside in burrows and prefer to live under leaf litter, forest debris, rocks, stream banks, and also in termite mounds Life Span: 5 to 8 years in captivity. Lifespan is likely shorter in the wild. Perils in the wild: Bats, birds, small mammals, large spiders, centipedes, large lizards and other scorpions Physical Adaptations: Eyesight of emperor scorpions is very poor. -

Redalyc.Tityus Zulianus and Tityus Discrepans Venoms Induced
Archivos Venezolanos de Farmacología y Terapéutica ISSN: 0798-0264 [email protected] Sociedad Venezolana de Farmacología Clínica y Terapéutica Venezuela Trejo, E.; Borges, A.; González de Alfonzo, R.; Lippo de Becemberg, I.; Alfonzo, M. J. Tityus zulianus and Tityus discrepans venoms induced massive autonomic stimulation in mice Archivos Venezolanos de Farmacología y Terapéutica, vol. 31, núm. 1, enero-marzo, 2012, pp. 1-5 Sociedad Venezolana de Farmacología Clínica y Terapéutica Caracas, Venezuela Available in: http://www.redalyc.org/articulo.oa?id=55923411003 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Tityus zulianus and Tityus discrepans venoms induced massive autonomic stimulation in mice E. Trejo, A. Borges, R. González de Alfonzo, I. Lippo de Becemberg and M. J. Alfonzo*. Cátedra de Patología General y Fisiopatología and Sección de Biomembranas. Instituto de Medicina Experimental. Facultad de Medicina. Universidad Central de Venezuela. Caracas. Venezuela. E.Trejo, Magister Scientiarum en Farmacología y Doctor en Bioquímica. R. González de Alfonzo, Doctora en Bioquímica, Biología Celular y Molecular. I. Lippo de Becemberg, Doctora en Medicina M. J. Alfonzo, Doctor en Bioquímica, Biología Celular y Molecular *Corresponding Author: Dr. Marcelo J. Alfonzo. Sección de Biomembranas. Instituto de Medicina Experimental. Facultad de Medicina Universidad Central de Venezuela. Ciudad Universitaria. Caracas. Venezuela. Teléfono: 0212-605-3654. fax: 058-212-6628877. Email: [email protected] Running title: Massive autonomic stimulation by Venezuelan scorpion venoms. Título corto: Estimulación autonómica masiva producida por los venenos de escorpiones venezolanos. -
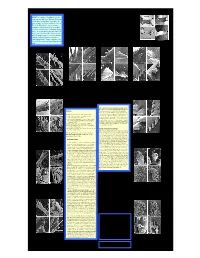
Scorpion Tufts.Pdf
ABSTRACT. Five scorpion genera of superfamily Iuroidea exhibit Tarsal Spinule Clusters and Evolution of the ancient disjunct ranges (South America, North America, Mediterranean), and are an important object in the study of scorpion phylogeny. They have an exceptional variety of tarsal leg setation/spination (Soleglad & Fet 2003). New SEM data from all five genera and two families: Superfamily Iuroidea (Scorpiones) Caraboctonidae (Caraboctonus, Hadruroides, Hadrurus) and Iuridae (Iurus, Calchas) are characterized in detail. We demonstrate two major patterns: (1) an irregular median row of grouped spinule clusters, found in juvenile to subadult but reduced in adult (Calchas); or (2) a median 1 2 3 4 row of highly concentrated spinule clusters. Pattern (2) is either forming Victor Fet , Michael E. Soleglad , David P.A. Neff & Iasmi Stathi “spinule tufts” (Caraboctonus, Hadruroides, Iurus), or individual 1Department of Biological Sciences, and 3Department of Chemistry, Marshall University, Huntington, WV, USA; “spinule-looking” protuberances (Hadrurus). We suggest that the latter Hadrurus obscurus are a derived feature as a result of fusion of separate spinules into a solid 2Borrego Springs, CA, USA; 3Department of Zoology, University of Crete, Irakleio, Crete, Greece structure. General appearance of tarsal armament in Iuroidea Figs. 17-20. Lateral-ventral view of leg III tarsus of Hadrurus a. arizonensis. 17. Figs. 21-24. Lateral-ventral view of leg III (leg IV in Fig. 24) tarsus of Mexican Figs. 25-28. Lateral-ventral view of leg III tarsus of Baja California Hadrurus species. Closeup of fused spinule cluster of adult (carapace length = xx.x mm). Borrego Hadrurus species. 21. Closeup of fused spinule cluster of adult H. -

Soleglad, Fet & Lowe: Hadrurus “Spadix” Subgroup 17
Soleglad, Fet & Lowe: Hadrurus “spadix” Subgroup 17 Figures 31–33 Comparisons of Hadrurus obscurus and H. spadix, metasomal segments II–III, ventral view, showing diagnostic setation located between the ventromedian (VM) carinae. 31. H. obscurus, male (pale phenotype, segment II length = 8.44 mm, segment III length = 9.26 mm), Bird Spring Canyon Road, Kern Co., California, USA. 32. H. obscurus, female (dark phenotype, segment II length = 5.02 mm, segment III length = 5.70 mm), Bird Spring Canyon Road, Kern Co., California, USA. 33. H. spadix, female (segment II length = 7.87 mm, segment III length = 8.36 mm), Apex Mine in Curly Hollow Wash, Washington Co., Utah, USA. 19). However, to support our suspicion, we have ex- As stated above the positions and numbers of chelal amined two H. obscurus specimens collected from the internal trichobothria are essentially identical in Ha- same locality where one has a carapace pattern of H. drurus obscurus and H. spadix, while both numbers and spadix and the other a pattern typical of H. obscurus (see positions differ in H. anzaborrego (see Fig. 19). H. Fig. 20 for color closeup images of these carapaces, and anzaborrego has three internal accessory trichobothria Figs. 9–10 for overall comparison with “arizonensis” whereas the other two species have two. Statistics group species). Based on these data, we suggest here that involving over 250 samples show that these numerical these carapacial pattern differences are analogous to differences are observed in over 87 % of the specimens those exhibited by the dark and pale phenotypes of H. examined (Fig. -

Arachnida Dictionnaire Des Noms Scientifiques Des
The electronic publication Arachnides - Bulletin de Terrariophile et de Recherche N°61 (2011) has been archived at http://publikationen.ub.uni-frankfurt.de/ (repository of University Library Frankfurt, Germany). Please include its persistent identifier urn:nbn:de:hebis:30:3-371887 whenever you cite this electronic publication. ARACHNIDES BULLETIN DE TERRARIOPHILIE ET DE RECHERCHES DE L’A.P.C.I. (Association Pour la Connaissance des Invertébrés) 61 2011 PREMIERES DONNEES SUR LA DIVERSITE SCORPIONIQUE DANS LA REGION DU SOUF (ALGERIE) Salah Eddine SADINE 1, Samia BISSAT 2 & Mohamed Didi OULD ELHADJ 1 [email protected] 1. Laboratoire de Protection des Écosystèmes en zones Arides et Semi-arides. Université KASDI Merbah-Ouargla. Algérie. BP 511 Route Ghardaïa – Ouargla. 30000. Algérie 2. Laboratoire Bio ressources. Université KASDI Merbah-Ouargla. Algérie. BP 511 Route Ghardaïa – Ouargla. 30000. Algérie ------------------------------------------------------------ Résumé : Le Souf est situé au Sud- Est de l’Algérie, aux confins septentrionaux du Grand Erg Oriental, entre les 33° et 34° de latitude Nord, et les 6° et 8° de longitude Est, touchant les frontières tunisienne et libyenne. Cette immense étendue sablonneuse abrite plusieurs faunes désertiques hautement diversifiées. Une étude originale sur la faune scorpionique dans cette région, nous a permis d’inventorier et identifier en totalité huit (08) espèces des scorpions, réparties d’une manière typique selon les différents biotopes naturels (Erg et reg) et biotopes anthropiques (Palmeraies ou oasis et milieux urbains). Une analyse factorielle des correspondances appliquées aux espèces trouvées nous a révélé que l’ Androctonus autralis est l’espèce omniprésente dans tous les biotopes et l’unique espèce qui fréquente les milieux urbains, Androctonus amoreuxi en deuxième place avec une large répartition qui fréquente la majorité des biotopes sauf le milieu urbain.