Soleglad, Fet & Lowe: Hadrurus “Spadix” Subgroup 17
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 It's All Geek to Me: Translating Names Of
IT’S ALL GEEK TO ME: TRANSLATING NAMES OF INSECTARIUM ARTHROPODS Prof. J. Phineas Michaelson, O.M.P. U.S. Biological and Geological Survey of the Territories Central Post Office, Denver City, Colorado Territory [or Year 2016 c/o Kallima Consultants, Inc., PO Box 33084, Northglenn, CO 80233-0084] ABSTRACT Kids today! Why don’t they know the basics of Greek and Latin? Either they don’t pay attention in class, or in many cases schools just don’t teach these classic languages of science anymore. For those who are Latin and Greek-challenged, noted (fictional) Victorian entomologist and explorer, Prof. J. Phineas Michaelson, will present English translations of the scientific names that have been given to some of the popular common arthropods available for public exhibits. This paper will explore how species get their names, as well as a brief look at some of the naturalists that named them. INTRODUCTION Our education system just isn’t what it used to be. Classic languages such as Latin and Greek are no longer a part of standard curriculum. Unfortunately, this puts modern students of science at somewhat of a disadvantage compared to our predecessors when it comes to scientific names. In the insectarium world, Latin and Greek names are used for the arthropods that we display, but for most young entomologists, these words are just a challenge to pronounce and lack meaning. Working with arthropods, we all know that Entomology is the study of these animals. Sounding similar but totally different, Etymology is the study of the origin of words, and the history of word meaning. -

Right-Sljp Offset of the Eocene Ballena River Valley Across the Elsinore Fault Zone, Southern California
http://dx.doi.org/10.7773/cm.v9i1.412 RIGHT-SLJP OFFSET OF THE EOCENE BALLENA RIVER VALLEY ACROSS THE ELSINORE FAULT ZONE, SOUTHERN CALIFORNIA Patrick L. Abbott Ronald P. Kies Dermis R. Kerr Department of GeologicaI Sciences San Diego State University San Diego, California 92182 U. S. A. ABSTRACT TWO small paleovalleys carved into granitic rocks of the Peninsular Ranges contain conglomerates similar to the Eocene Ballena Gravels farther west in San Diego County. These conglomerates are dominated by the distinctive, temporally restricted, Poway rhyolite clasts. The newly discovered fluvial conglomerates lie in the Vallecito Mountains east of the Elsinore fault zone. Extrapolation of the offset fluvial valley trends to the Elsinore fault suggests 37 kilometers (23 miles) of right slip. RESUMEN Dos valles pequeños paleolíticos, grabados en cuerpos graníticos de la Cor- dillera Peninsular, contienen conglomerados semejantes a las gravas de la Forma- ción Ballena del Eoceno, localizada al peste, en el Condado de San Diego. Este conglomerado es dominado por clastos riolíticos de la Formación Poway, los cuales son característicos y están temporalmente restringidos. Los conglomerados fluviales recientemente descubiertos, se encuentran en las Montanas del Vallecito, al este de la zona de la falla de Elsinore. Proyectando los rumbos de los conglome- rados fluviales y de las gravas a través de la falla de Elsinore, se observa un despla- zamiento de 37 km. (23 millas) en un sentido lateral-derecho. INTRODUCTION The Ballena Gravels (actually conglomerate ) were named by Fairbanks (1893) for a prominent, southwest-trending line of outcrops on the western flank of the batholithic Peninsular Ranges in San Diego County (figure 1). -

Anza-Borrego Desert State Park Bibliography Compiled and Edited by Jim Dice
Steele/Burnand Anza-Borrego Desert Research Center University of California, Irvine UCI – NATURE and UC Natural Reserve System California State Parks – Colorado Desert District Anza-Borrego Desert State Park & Anza-Borrego Foundation Anza-Borrego Desert State Park Bibliography Compiled and Edited by Jim Dice (revised 1/31/2019) A gaggle of geneticists in Borrego Palm Canyon – 1975. (L-R, Dr. Theodosius Dobzhansky, Dr. Steve Bryant, Dr. Richard Lewontin, Dr. Steve Jones, Dr. TimEDITOR’S Prout. Photo NOTE by Dr. John Moore, courtesy of Steve Jones) Editor’s Note The publications cited in this volume specifically mention and/or discuss Anza-Borrego Desert State Park, locations and/or features known to occur within the present-day boundaries of Anza-Borrego Desert State Park, biological, geological, paleontological or anthropological specimens collected from localities within the present-day boundaries of Anza-Borrego Desert State Park, or events that have occurred within those same boundaries. This compendium is not now, nor will it ever be complete (barring, of course, the end of the Earth or the Park). Many, many people have helped to corral the references contained herein (see below). Any errors of omission and comission are the fault of the editor – who would be grateful to have such errors and omissions pointed out! [[email protected]] ACKNOWLEDGEMENTS As mentioned above, many many people have contributed to building this database of knowledge about Anza-Borrego Desert State Park. A quantum leap was taken somewhere in 2016-17 when Kevin Browne introduced me to Google Scholar – and we were off to the races. Elaine Tulving deserves a special mention for her assistance in dealing with formatting issues, keeping printers working, filing hard copies, ignoring occasional foul language – occasionally falling prey to it herself, and occasionally livening things up with an exclamation of “oh come on now, you just made that word up!” Bob Theriault assisted in many ways and now has a lifetime job, if he wants it, entering these references into Zotero. -

Target-Specificity in Scorpions
Toxins 2017, 9, 312, doi: 10.3390/toxins9100312 S1 of S3 Supplementary Materials: Target‐specificity in scorpions; Comparing lethality of scorpion venoms across arthropods and vertebrates Arie van der Meijden, Bjørn Koch, Tom van der Valk, Leidy J. Vargas‐Muñoz and Sebastian Estrada‐Gómez Table S1. Table with GenBank CO1 accession numbers. Voucher numbers refer to the specimens in the collection of CIBIO/InBio at the University of Porto. Species Voucher Accession number Androctonus australis Sc904 gi379647585 Leiurus quinquestriatus Sc1062 gi379647601 Buthus ibericus Sc112 gi290918312 Grosphus flavopiceus Sc1085 gi379647593 Centruroides gracilis gi71743793 Heterometrus laoticus Sc1084 gi555299473 Pandinus imperator Sc1050 gi379647587 Hadrurus arizonensis Sc1042 gi379647597 Iurus kraepelini Sc866 gi379647589 Table S2. Mean dry venom compound per individual. Several milkings were made per (sub)adult specimen in some cases. Species n Dry venom (mg) mg/milking Androctonus australis 21 23.5 1.12 Leiurus quinquestriatus 51 25.1 0.49 Buthus ibericus 47 41.6 0.89 Centruroides gracilis 42 22.5 0.54 Babycurus jacksoni 38 53.9 1.42 Grosphus grandidieri 19 103.9 5.47 Hadrurus arizonensis 9 75.3 8.37 Iurus sp.* 12 35.2 2.93 Heterometrus laoticus 21 119 5.67 Pandinus imperator 15 72.7 4.85 *2 I. dufoureius, 6 I. kraepelini, 4 I. sp. Toxins 2017, 9, 312, doi: 10.3390/toxins9100312 S2 of S3 Table S3. Toxicological analysis of the venom of Grosphus grandidieri. “Toxic” means that the mice showed symptoms such as: pain, piloerection, excitability, salivation, lacrimation, dyspnea, diarrhea, temporary paralysis, but recovered within 20 h. “Lethal” means that the mice showed some or all the symptoms of intoxication and died within 20 h after injection. -

Spider and Scorpion Case
Spider and Scorpion case Black widow spider (Lactrodectus hesperus) Black widows are notorious spiders identified by the colored, hourglass-shaped mark on their abdomens. Several species answer to the name, and they are found in temperate regions around the world. This spider's bite is much feared because its venom is reported to be 15 times stronger than a rattlesnake's. In humans, bites produce muscle aches, nausea, and a paralysis of the diaphragm that can make breathing difficult; however, contrary to popular belief, most people who are bitten suffer no serious damage—let alone death. But bites can be fatal—usually to small children, the elderly, or the infirm. Fortunately, fatalities are fairly rare; the spiders are nonaggressive and bite only in self-defense, such as when someone accidentally sits on them. These spiders spin large webs in which females suspend a cocoon with hundreds of eggs. Spiderlings disperse soon after they leave their eggs, but the web remains. Black widow spiders also use their webs to ensnare their prey, which consists of flies, mosquitoes, grasshoppers, beetles, and caterpillars. Black widows are comb- footed spiders, which means they have bristles on their hind legs that they use to cover their prey with silk once it has been trapped. To feed, black widows puncture their insect prey with their fangs and administer digestive enzymes to the corpses. By using these enzymes, and their gnashing fangs, the spiders liquefy their prey's bodies and suck up the resulting fluid. Giant desert hairy scorpion (Hadrurus arizonensis) Hadrurus arizonensis is distributed throughout the Sonora and Mojave deserts. -
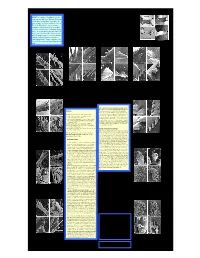
Scorpion Tufts.Pdf
ABSTRACT. Five scorpion genera of superfamily Iuroidea exhibit Tarsal Spinule Clusters and Evolution of the ancient disjunct ranges (South America, North America, Mediterranean), and are an important object in the study of scorpion phylogeny. They have an exceptional variety of tarsal leg setation/spination (Soleglad & Fet 2003). New SEM data from all five genera and two families: Superfamily Iuroidea (Scorpiones) Caraboctonidae (Caraboctonus, Hadruroides, Hadrurus) and Iuridae (Iurus, Calchas) are characterized in detail. We demonstrate two major patterns: (1) an irregular median row of grouped spinule clusters, found in juvenile to subadult but reduced in adult (Calchas); or (2) a median 1 2 3 4 row of highly concentrated spinule clusters. Pattern (2) is either forming Victor Fet , Michael E. Soleglad , David P.A. Neff & Iasmi Stathi “spinule tufts” (Caraboctonus, Hadruroides, Iurus), or individual 1Department of Biological Sciences, and 3Department of Chemistry, Marshall University, Huntington, WV, USA; “spinule-looking” protuberances (Hadrurus). We suggest that the latter Hadrurus obscurus are a derived feature as a result of fusion of separate spinules into a solid 2Borrego Springs, CA, USA; 3Department of Zoology, University of Crete, Irakleio, Crete, Greece structure. General appearance of tarsal armament in Iuroidea Figs. 17-20. Lateral-ventral view of leg III tarsus of Hadrurus a. arizonensis. 17. Figs. 21-24. Lateral-ventral view of leg III (leg IV in Fig. 24) tarsus of Mexican Figs. 25-28. Lateral-ventral view of leg III tarsus of Baja California Hadrurus species. Closeup of fused spinule cluster of adult (carapace length = xx.x mm). Borrego Hadrurus species. 21. Closeup of fused spinule cluster of adult H. -

Euscorpius. 2009
Euscorpius Occasional Publications in Scorpiology Courtship and Mating in Heterometrus petersii (Thorell, 1876) (Scorpiones: Scorpionidae) Guo-Bin Jiao & Ming-Sheng Zhu June 2009 – No. 84 Euscorpius Occasional Publications in Scorpiology EDITOR: Victor Fet, Marshall University, ‘[email protected]’ ASSOCIATE EDITOR: Michael E. Soleglad, ‘[email protected]’ Euscorpius is the first research publication completely devoted to scorpions (Arachnida: Scorpiones). Euscorpius takes advantage of the rapidly evolving medium of quick online publication, at the same time maintaining high research standards for the burgeoning field of scorpion science (scorpiology). Euscorpius is an expedient and viable medium for the publication of serious papers in scorpiology, including (but not limited to): systematics, evolution, ecology, biogeography, and general biology of scorpions. Review papers, descriptions of new taxa, faunistic surveys, lists of museum collections, and book reviews are welcome. Derivatio Nominis The name Euscorpius Thorell, 1876 refers to the most common genus of scorpions in the Mediterranean region and southern Europe (family Euscorpiidae). Euscorpius is located on Website ‘http://www.science.marshall.edu/fet/euscorpius/’ at Marshall University, Huntington, WV 25755-2510, USA. The International Code of Zoological Nomenclature (ICZN, 4th Edition, 1999) does not accept online texts as published work (Article 9.8); however, it accepts CD-ROM publications (Article 8). Euscorpius is produced in two identical versions: online (ISSN 1536-9307) -

Defensive Behavior Is Associated with Morphology and Performance in Scorpions
Choose Your Weapon: Defensive Behavior Is Associated with Morphology and Performance in Scorpions Arie van der Meijden1*, Pedro Lobo Coelho1, Pedro Sousa1, Anthony Herrel2 1 CIBIO, Centro de Investigac¸a˜o em Biodiversidade e Recursos Gene´ticos, Campus Agra´rio de Vaira˜o, Vaira˜o, Portugal, 2 UMR 7179, Muse´um National d9Histoire Naturelle, De´partement d9Ecologie et de Gestion de la Biodiversite´, Paris, France Abstract Morphology can be adaptive through its effect on performance of an organism. The effect of performance may, however, be modulated by behavior; an organism may choose a behavioral option that does not fully utilize its maximum performance. Behavior may therefore be decoupled from morphology and performance. To gain insight into the relationships between these levels of organization, we combined morphological data on defensive structures with measures of defensive performance, and their utilization in defensive behavior. Scorpion species show significant variation in the morphology and performance of their main defensive structures; their chelae (pincers) and the metasoma (‘‘tail’’) carrying the stinger. Our data show that size-corrected pinch force varies to almost two orders of magnitude among species, and is correlated with chela morphology. Chela and metasoma morphology are also correlated to the LD50 of the venom, corroborating the anecdotal rule that dangerously venomous scorpions can be recognized by their chelae and metasoma. Analyses of phylogenetic independent contrasts show that correlations between several aspects of chela and metasoma morphology, performance and behavior are present. These correlations suggest co-evolution of behavior with morphology and performance. Path analysis found a performance variable (pinch force) to partially mediate the relationship between morphology (chela aspect ratio) and behavior (defensive stinger usage). -

Scorpion Phylogeography in the North American Aridlands
UNLV Theses, Dissertations, Professional Papers, and Capstones 8-1-2012 Scorpion Phylogeography in the North American Aridlands Matthew Ryan Graham University of Nevada, Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Biology Commons, Desert Ecology Commons, and the Population Biology Commons Repository Citation Graham, Matthew Ryan, "Scorpion Phylogeography in the North American Aridlands" (2012). UNLV Theses, Dissertations, Professional Papers, and Capstones. 1668. http://dx.doi.org/10.34917/4332649 This Dissertation is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/or on the work itself. This Dissertation has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. SCORPION PHYLOGEOGRAPHY IN THE NORTH AMERICAN ARIDLANDS by Matthew Ryan Graham Bachelor of Science Marshall University 2004 Master of Science Marshall University 2007 A dissertation submitted in partial fulfillment of the requirements for the Doctor of Philosophy in Biological Sciences School of Life Sciences College of Sciences The Graduate College University of Nevada, Las Vegas August 2012 Copyright by Matthew R. Graham, 2012 All Rights Reserved THE GRADUATE COLLEGE We recommend the thesis prepared under our supervision by Matthew R. -

Beck's Desert Scorpion
Molecular Phylogenetics and Evolution 69 (2013) 502–513 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogeography of Beck’s Desert Scorpion, Paruroctonus becki, reveals Pliocene diversification in the Eastern California Shear Zone and postglacial expansion in the Great Basin Desert ⇑ Matthew R. Graham a, , Jef R. Jaeger a, Lorenzo Prendini b, Brett R. Riddle a a School of Life Sciences, University of Nevada Las Vegas, 4505 South Maryland Parkway, Las Vegas, NV 89154-4004, USA b Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024-5192, USA article info abstract Article history: The distribution of Beck’s Desert Scorpion, Paruroctonus becki (Gertsch and Allred, 1965), spans the Received 12 November 2012 ‘warm’ Mojave Desert and the western portion of the ‘cold’ Great Basin Desert. We used genetic analyses Revised 10 July 2013 and species distribution modeling to test whether P. becki persisted in the Great Basin Desert during the Accepted 29 July 2013 Last Glacial Maximum (LGM), or colonized the area as glacial conditions retreated and the climate Available online 9 August 2013 warmed. Phylogenetic and network analyses of mitochondrial cytochrome c oxidase 1 (cox1), 16S rDNA, and nuclear internal transcribed spacer (ITS-2) DNA sequences uncovered five geographically-structured Keywords: groups in P. becki with varying degrees of statistical support. Molecular clock estimates and the geograph- Biogeography ical arrangement of three of the groups suggested that Pliocene geological events in the tectonically Basin and range COI dynamic Eastern California Shear Zone may have driven diversification by vicariance. -

Scorpions Entire Body Behaving Like an Eye?
Scorpions Entire Body Even with the shock of this creature slinking is merry Behaving like an Eye? way across her foot, the woman in her heightened surprise did not panic but rather picked it up with a By: Carissa Hurdstrom scrap piece of paper and put it in a bowl on the table. She ran to the back of the house to dig around in a closet, moments later bouncing down the hall with a small black light, quickly plugged it in and held it over the scorpion. It began to glow brilliantly with a cyan-green color. This kind of house warming greeting is common for the residents of the southwestern United States, since many of the now discovered 1,500 species of Scorpi- ons live in desert climates and are typically perceived as poisonous pests that must be exterminated. Often exterminators will use a black light to scope out the location for our perceivably ominous friend, but how does this work? The common black light seen at Halloween parties are an emission source of Ultraviolet light that are often made from specially designed florescent or mercury vapor lamps. Ultraviolet light itself is outside the visible rage of electromagnetic radiation On a fairly warm afternoon in a (light) that we humans can see. From the electrometric small town near the northern border Spectrum diagram, Ultraviolet waves range in of Arizona, a woman sat down at her home computer to amplitudes from about 10–400 nm. Humans can only type away in response to some emails. Only a few minutes see wavelengths from approximately 400–720 nm. -

Peninsular Bighorn Sheep Recovery 2011 Annual Report
CALIFORNIA DEPARTMENT OF FISH AND GAME PENINSULAR BIGHORN SHEEP RECOVERY 2011 ANNUAL REPORT A cooperative effort by the California Department of Fish and Game, U.S. Fish and Wildlife Service, and California Department of Parks and Recreation Photo By Janene Colby This report presents information on the status, distribution, and management of peninsular bighorn sheep in eastern San Diego County and portions of Riverside and Imperial Counties, California, from January 1, 2011 through December 31, 2011. Report prepared by Janene Colby and Randy Botta TABLE OF CONTENTS SUMMARY...…………………………………………………………………………… 2 PROJECT PERSONNEL……………………………………………………………… 3 CDFG Resource Management and Air Services Divisions…........................... 3 CDFG South Coast Region………………………….……..……………...…… 3 RECOVERY PROGRAM OVERVIEW Telemetry Monitoring…..……………………….………….……………..…… 4 Population Size and Estimation…...………………………….…………..……. 5 Distribution and Movement………….…………………………………...…..... 7 Central Santa Rosa Mountains…………………………………….....…... 7 Southern Santa Rosa Mountains…………..……………………...……… 7 Coyote Canyon........................................................................................... 7 Northern San Ysidro Mountains……………………..…………...……… 9 Southern San Ysidro Mountains…………………….…………...………11 Vallecito Mountains.....……………………………………..……...…….13 Carrizo Canyon…...…………………………………………………….. 15 Survivorship….…………………………………...………………………..….. 17 Lamb Mortality Monitoring………..……………………………………...…. 19 2012 PROPOSED ACTIVITIES …………………………………………………... 19 1 SUMMARY This report