06. Inotropes (F Schneider)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heart Rate Information Sheet Finding Your Pulse Taking Your Pulse
Heart rate In this activity you will measure your heart rate and investigate the effect that other things have on your heart rate. Information sheet You can measure your heart (pulse) rate anywhere on your body where a major artery is close to the surface of your skin. The easiest places are: • on the front of your forearm • just above your wrist on your thumb side • on the side of your neck about half way between your chin and your ear. Finding your pulse Try to locate your pulse in one of these places, using the tips of your index and middle fingers. You should feel a gentle, regular beat. This is your heart rate. Do not use your thumb, as your thumb has a pulse of its own. Taking your pulse When you find your pulse, use a stopwatch or a watch with a second hand to count how many beats there are in a full minute (60 seconds). In most situations, taking your pulse rate over one minute will give a reasonably accurate result, but if you want to take your pulse after exercise, you should do so over a much shorter time interval. After exercise your pulse rate will be changing rapidly. To get a reasonably accurate result, start to measure the rate immediately after the exercise and count the number of beats in 10 seconds. Multiply this number by six to find your heart rate. For an adult, a normal resting heart rate is between 60–100 beats a minute. The fitter you are, the lower your resting heart beat will be. -

Heart to Heart - STEAM Activity
Heart to Heart - STEAM Activity Purpose: The main objective of this exercise is to introduce how the human heart works. This lesson will be divided into four different lessons, including an introduction to heart anatomy, heart beats and pulses, and the circulatory system. We will be using coloring activities, stethoscopes, and handmade pumps to reinforce the concepts seen in this lesson. Vocabulary ● Artery: A blood vessel that carries blood high in oxygen content away from the heart to the farthest reaches of the body. ● Vein: a Blood vessel that carries blood low in oxygen content from the body back to the heart. ● Atrium: One of the two upper cavities of the heart that passes blood to the ventricles. ● Ventricles: One of the two lower chambers of the heart that receives blood from the atria. ● Valves: Tissue-paper thin membranes attached to the heart wall that constantly open and close to regulate blood flow. ● Pulse: A rhythmical, mechanical throbbing of the arteries as blood pumps through them. ● Heart Rate: The number of times per minute that the heart contracts - the number of heart beats per minute (bpm). ● Taquicardia: A high resting heart rate that is usually higher than 100 beats per minute. ● Bradycardia: A low resting heart rate that is usually lower than 60 beats per minute. ● Circulation: The movement of blood through the vessels of the body by the pumping action of the heart. It distributes nutrients and oxygen and removes waste products from all parts of the body. ● Pulmonary Circulation: The portion of the circulatory system that carries deoxygenated blood from the heart to the lungs and oxygenated blood back to the heart. -

Chronotropic Incompetence: a Proposal for Br Heart J: First Published As 10.1136/Hrt.70.5.400 on 1 November 1993
400 Br Heart3' 1993;70:400-402 FOR DEBATE Chronotropic incompetence: a proposal for Br Heart J: first published as 10.1136/hrt.70.5.400 on 1 November 1993. Downloaded from definition and diagnosis Demosthenes Katritsis, A John Camm Between 1958 and 1960 Astrand docu- exercise testing that is <75%16 or <80%"7 of mented the normal heart rate response to the predicted MPHR. Other arbitrary defini- exercise in healthy individuals and noted that tions such as a maximum exercise heart rate the maximum heart rate decreased with age.' 2 <100 beats/min" or <120 beats/min'8 have A reduced cardiac chronotropic response to also been used. However, the achievement of isoprenaline has been reported in elderly sub- maximum exercise is not always possible. jects and alterations in catecholamine- Elderly cardiac patients, especially those adrenergic receptor interactions may be disabled by chronotropic incompetence, are responsible for this change of cardiovascular unable to perform sufficient exercise on the regulation in the elderly.35 In addition the treadmill. Furthermore, everyday life activi- parasympathetic innervation of the sinus ties of these patients usually correspond to node is currently under investigation.6 Over low work loads up to 6 metabolic equivalents the years it has also been recognised that corresponding to the first stage of the stan- an inadequate chronotropic response dard Bruce protocol. In addition, not much is (chronotropic incompetence) at maximal known about the patterns of heart rate accel- exercise is common in patients with cardiac eration and deceleration in the presence of disease78 and much interest has centred chronotropic incompetence. -

Toolbox-Talks--Blood-Pressure.Pdf
TOOLBOX Toolbox Talk #1 TALKS Blood Pressure vs. Heart Rate While your blood pressure is the force of your blood moving through your blood vessels, your heart rate is the number of times your heart beats per minute. They are two separate measurements and indicators of health. • For people with high blood pressure (HBP or hypertension), there’s no substitute for measuring blood pressure. • Heart rate and blood pressure do not necessarily increase at the same rate. A rising heart rate does not cause your blood pressure to increase at the same Quarter: rate. Even though your heart is beating more times a minute, healthy blood BLOOD vessels dilate (get larger) to allow more blood to flow through more easily. PRESSURE When you exercise, your heart speeds up so more blood can reach your muscles. It may be possible for your heart rate to double safely, while your blood pressure may respond by only increasing a modest amount. Talk Number: Heart Rate and Exercise 1 In discussions about high blood pressure, you will often see heart rate Blood mentioned in relation to exercise. Your target heart rate is based on age and Pressure can help you monitor the intensity of your exercise. vs. • If you measure your heart rate (take your pulse) before, during and after Heart Rate physical activity, you’ll notice it will increase over the course of the exercise. • The greater the intensity of the exercise, the more your heart rate will increase. • When you stop exercising, your heart rate does not immediately return to your normal (resting) heart rate. -

Electrocardiography in Horses – Part 2: How to Read the Equine ECG
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Ghent University Academic Bibliography Vlaams Diergeneeskundig Tijdschrift, 2010, 79 Thema: electrocardiography in horses 337 Electrocardiography in horses – part 2: how to read the equine ECG Elektrocardiografie bij paarden – deel 2: interpretatie van het EKG T. Verheyen, A. Decloedt, D. De Clercq, P. Deprez, S. U. Sys, G. van Loon Department of Large Animal Internal Medicine Faculty of Veterinary Medicine, Ghent University Salisburylaan 133, 9820 Merelbeke, Belgium [email protected] ABSTRACT The equine practitioner is faced with a wide variety of dysrhythmias, of which some are physiological. The recording of an exercise electrocardiogram (ECG) can help distinguish between physiological and patholog- ical dysrhythmias, underlining the importance of exercise recordings. The evaluation of an ECG recording should be performed in a highly methodical manner in order to avoid errors. Each P wave should be followed by a QRS complex, and each QRS complex should be preceded by a P wave. The classification of dysrhythmias according to their origin helps to understand the associated changes on the ECG. In this respect, sinoatrial nodal (SA nodal), atrial myocardial, atrioventricular nodal (AV nodal) and ventricular myocardial dysrhythmias can be distinguished. Artefacts on the ECG can lead to misinterpretations. Recording an ECG of good quality is a prerequisite to prevent misinterpretations, but artefacts are almost impossible to avoid when recording during exercise. Changes in P or T waves during exercise also often lead to misinterpretations, however they have no clinical significance. SAMENVATTING De paardendierenarts wordt geconfronteerd met een waaier van dysritmieën, waarvan sommige fysiologisch zijn. -

Presentation Title
Meet the experts: Cardiogenic Shock Inotropes: effects on the heart, the microcirculation and other organs ACCA Masterclass 2017 Alessandro Sionis Director Acute & Intensive Cardiac Care Unit Hospital de la Santa Creu I Sant Pau Universitat de Barcelona Spain Disclosures (last 5 years) ► Speaker: Abiomed, Maquet, Novartis, Orion-Pharma ► Clinical trials: Cardiorentis, Novartis, Orion-Pharma ► Research grants: Novartis, Orion-Pharma ► Royalties: No PATIENT WITH AHF Bedside assessment to identify haemodynamic profile CONGESTION? YES (95% of AHF patients) NO (5% of AHF patients) “Wet” “Dry” POOR PERFUSION? NO YES NO YES “Wet” & “Warm” “Wet” & “Cold” “Dry” & “Warm” “Dry” & “Cold” Adapted from 2016 ESC HF Guildeines PATIENT WITH AHF Bedside assessment to identify haemodynamic profile CONGESTION? YES (95% of AHF patients) NO (5% of AHF patients) “Wet” “Dry” POOR PERFUSION? NO YES NO YES “Wet” & “Warm” “Wet” & “Cold” “Dry” & “Warm” “Dry” & “Cold” Adapted from 2016 ESC HF Guildeines Definitions of Terms Used in Cardiogenic Shock Diagnosis Term Definition Symptoms/signs of congestion (left-sided) Orthopnoea, paroxysmal nocturnal dyspnoea, pulmonary rales (bilateral), peripheral oedema (bilateral). Symptoms/signs of congestion (right-sided) Jugular venous dilatation, peripheral oedema, congested hepatpmegaly, hepatojugular reflux, ascites, symptoms of gut congestionsymptoms of gut congestion. Symptoms/signs of hypoperfusion Clinical: cold sweated extremities, oliguria, mental confusion, dizziness, narrow pulse pressure. Laboratory measures: metabolic -

Nitric Oxide, the Biological Mediator of the Decade: Fact Or Fiction?
Eur Respir J 1997; 10: 699–707 Copyright ERS Journals Ltd 1997 DOI: 10.1183/09031936.97.10030699 European Respiratory Journal Printed in UK - all rights reserved ISSN 0903 - 1936 SERIES 'CLINICAL PHYSIOLOGY IN RESPIRATORY INTENSIVE CARE' Edited by A. Rossi and C. Roussos Number 14 in this Series Nitric oxide, the biological mediator of the decade: fact or fiction? S. Singh, T.W. Evans Nitric oxide, the biological mediator of the decade: fact or fiction? S. Singh, T.W. Evans. Unit of Critical Care, National Heart & ERS Journals Ltd 1997. Lung Institute, Royal Brompton Hospital, ABSTRACT: Nitric oxide (NO), an atmospheric gas and free radical, is also an London, UK. important biological mediator in animals and humans. Its enzymatic synthesis by constitutive (c) and inducible (i) isoforms of NO synthase (NOS) and its reactions Correspondence: T.W. Evans with other biological molecules such as reactive oxygen species are well charac- Royal Brompton Hospital Sydney Street terized. NO modulates pulmonary and systemic vascular tone through its vasodila- London SW3 6NP tor property. It has antithrombotic functions and mediates some consequences of UK the innate and acute inflammatory responses; cytokines and bacterial toxins induce widespread expression of iNOS associated with microvascular and haemodynam- Keywords: Acute respiratory distress syn- ic changes in sepsis. drome Within the lungs, a diminution of NO production is implicated in pathological hypoxic pulmonary vasoconstriction states associated with pulmonary hypertension, such as acute respiratory distress nitric oxide syndrome: inhaled NO is a selective pulmonary vasodilator and can improve ven- nitric oxide synthase tilation-perfusion mismatch. However, it may have deleterious effects through mod- pulmonary hypertension ulating hypoxic pulmonary vasoconstriction. -

Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G
& Experim l e ca n i t in a l l C Aliya, J Clinic Experiment Cardiol 2011, 2:11 C f a Journal of Clinical & Experimental o r d l DOI: 10.4172/2155-9880.1000170 i a o n l o r g u y o J Cardiology ISSN: 2155-9880 Review Article Open Access Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G. College, Kakatiya University, Warangal, India Abstract Heart is one of the most important organs present in human body which pumps blood throughout the body using blood vessels. With each heartbeat, blood is sent throughout the body, carrying oxygen and nutrients to all the cells in body. The cardiac cycle is the sequence of events that occurs when the heart beats. Blood pressure is maximum during systole, when the heart is pushing and minimum during diastole, when the heart is relaxed. Vasodilation caused by relaxation of smooth muscle cells in arteries causes an increase in blood flow. When blood vessels dilate, the blood flow is increased due to a decrease in vascular resistance. Therefore, dilation of arteries and arterioles leads to an immediate decrease in arterial blood pressure and heart rate. Cardiac output is the amount of blood ejected by the left ventricle in one minute. Cardiac output (CO) is the volume of blood being pumped by the heart, by left ventricle in the time interval of one minute. The effects of vasodilation, how the blood quantity increases and decreases along with the blood flow and the arterial blood flow and resistance on cardiac output is discussed in this reviewArticle. -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -

Jugular Venous Pressure
NURSING Jugular Venous Pressure: Measuring PRACTICE & SKILL What is Measuring Jugular Venous Pressure? Measuring jugular venous pressure (JVP) is a noninvasive physical examination technique used to indirectly measure central venous pressure(i.e., the pressure of the blood in the superior and inferior vena cava close to the right atrium). It is a part of a complete cardiovascular assessment. (For more information on cardiovascular assessment in adults, see Nursing Practice & Skill ... Physical Assessment: Performing a Cardiovascular Assessment in Adults ) › What: Measuring JVP is a screening mechanism to identify abnormalities in venous return, blood volume, and right heart hemodynamics › How: JVP is determined by measuring the vertical distance between the sternal angle and the highest point of the visible venous pulsation in the internal jugular vein orthe height of the column of blood in the external jugular vein › Where: JVP can be measured in inpatient, outpatient, and residential settings › Who: Nurses, nurse practitioners, physician assistants, and treating clinicians can measure JVP as part of a complete cardiovascular assessment What is the Desired Outcome of Measuring Jugular Venous Pressure? › The desired outcome of measuring JVP is to establish the patient’s JVP within the normal range or for abnormal JVP to be identified so that appropriate treatment may be initiated. Patients’ level of activity should not be affected by having had the JVP measured ICD-9 Why is Measuring Jugular Venous Pressure Important? 89.62 › The JVP is -
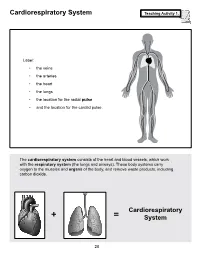
Cardiorespiratory System Teaching Activity 1
Cardiorespiratory System Teaching Activity 1. Label: • the veins • the arteries • the heart • the lungs • the location for the radial pulse • and the location for the carotid pulse. The cardiorespiratory system consists of the heart and blood vessels, which work with the respiratory system (the lungs and airways). These body systems carry oxygen to the muscles and organs of the body, and remove waste products, including carbon dioxide. Cardiorespiratory + = System 28 The Most Important Muscle: The Heart Teaching Activity 1, continued. In the circulatory system, the heart is a muscle that acts as a pump. In fact, the heart is a double pump. Blood that needs oxygen enters the heart and is pumped by the first pump to the lungs. The second pump of the heart pumps the oxygen-rich blood to all the other parts of the body. This gives the heart its common, “lub-dub” sound. The number of times the heart pumps, or beats, is counted in a minute. This is known as the pulse rate. A person’s pulse is affected by their current level of activity. If you are sleeping or do no physical activity at all, your heart is pumping at a resting heart rate. When active, you are using all your body systems. These systems require fuel in the form of calories (found in food), and oxygen (what you breathe). The more active you are, the Hearty Facts more fuel your muscles need. This is why your breathing rate and your heart rate increase when • Your system of blood vessels (arteries, you exercise. veins, and capillaries) is over 60,000 miles long. -

Cardiovascular System: Heart
Cardiovascular System: Heart Cardiovascular System – Heart Conducting cells: Cardiac Electrophysiology Cardiac cells specialized to quickly spread action potentials across myocardium • Weak force generators System allows for orderly, sequential depolarization and Intrinsic Conduction System: contraction of heart Normal sinus rhythm: 1) AP originates at SA node 2) SA node fires at 60 – 100 beats / min Atrial internodal tracts Sinoatrial node: (SA node) 3) Correct myocardial activation sequence • Located in right atrial wall • Initiates action potentials (APs) • Pacemaker (~ 80 beats / min) Bundle branches Atrioventricular node: (AV Node) • Connects atria to ventricles • Slowed conduction velocity • Ventricular filling Purkinje Bundle of His fibers Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 18.14 1 Cardiovascular System – Heart Cardiac Electrophysiology The autonomic nervous system can directly affect the heart rate; these effects are called chronotropic effects Recall: spontaneous depolarization = VG Na+ channels Positive chronotrophic effects: (increase heart rate) • Under sympathetic control Leads to g ; cells SA Na NE node reach threshold more rapidly Sinoatrial node Pharmacology: β1 receptors β-blockers (e.g., propanolol) Negative chronotrophic effects: (decrease heart rate) • Under parasympathetic control Leads to gNa; cells reach threshold SA less rapidly ACh node Leads to gK; cells hyperpolarized during Muscarinic receptors repolarization stage (further from threshold) Costanzo (Physiology, 4th ed.) – Figure