Desmin Is a Specific Marker for Rhabdomyosarcomas of Human and Rat Origin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Desmin Interacts Directly with Mitochondria
International Journal of Molecular Sciences Article Desmin Interacts Directly with Mitochondria Alexander A. Dayal 1, Natalia V. Medvedeva 1, Tatiana M. Nekrasova 1, Sergey D. Duhalin 1, Alexey K. Surin 1,2 and Alexander A. Minin 1,* 1 Institute of Protein Research of Russian Academy of Sciences, Vavilova st., 34, 119334 Moscow, Russia; [email protected] (A.A.D.); [email protected] (N.V.M.); [email protected] (T.M.N.); [email protected] (S.D.D.); [email protected] (A.K.S.) 2 Pushchino Branch, Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Prospekt Nauki 6, Pushchino, 142290 Moscow Region, Russia * Correspondence: [email protected] Received: 14 October 2020; Accepted: 26 October 2020; Published: 30 October 2020 Abstract: Desmin intermediate filaments (IFs) play an important role in maintaining the structural and functional integrity of muscle cells. They connect contractile myofibrils to plasma membrane, nuclei, and mitochondria. Disturbance of their network due to desmin mutations or deficiency leads to an infringement of myofibril organization and to a deterioration of mitochondrial distribution, morphology, and functions. The nature of the interaction of desmin IFs with mitochondria is not clear. To elucidate the possibility that desmin can directly bind to mitochondria, we have undertaken the study of their interaction in vitro. Using desmin mutant Des(Y122L) that forms unit-length filaments (ULFs) but is incapable of forming long filaments and, therefore, could be effectively separated from mitochondria by centrifugation through sucrose gradient, we probed the interaction of recombinant human desmin with mitochondria isolated from rat liver. Our data show that desmin can directly bind to mitochondria, and this binding depends on its N-terminal domain. -

In Situlocalization of Cytoskeletal Elements in the Human Trabecular
Investigative Ophthalmology & Visual Science. Vol. 31. No. 9. September 1990 Cops right £• Association lor Research in Vision and Ophthalmology In Situ Localization of Cytoskeletal Elements in the Human Trabecular Meshwork and Cornea Robert N. Weinreb* and Mark I. Ryderf The authors compared cytoskeletal elements of the in situ human trabccular-mcshwork cell with in situ human corneal cells using indirect immunofluorcsccncc staining for tubulin and intermediate filaments (vimentin, cytokeratin, and desmin) and NBD-phallacidin staining for f-actin using both fixed frozen and unfixed frozen sections from postmortem eyes. Both f-actin and tubulin were found throughout the cell body of trabecular-meshwork cells, keratocytes, corneal endothelium, and corneal epithelium. The f-actin staining pattern was concentrated at the cell periphery of these four cell types. Vimentin stain was intensely localized in focal areas of the trabecular-meshwork cell, keratocytes, and throughout the corneal cndothelium. A general anticytokeratin antibody was intensely localized in corneal epithelium and endothelium. However, PKK-1 anticytokeratin antibody was seen only in superficial layers of corneal epithelium and not in corneal endothelium. The 4.62 anticytokeratin antibody was not observed in either corneal epithelium or endothelium. None of these three cytokera- tin antibodies were seen in trabccular-mcshwork cells or keratocytes. Desmin stain was not noted in any of these cell types. In general, cytoskeletal staining of unfixed frozen sections showed a similar staining pattern for f-actin and tubulin but a more uniform and intense staining pattern for vimentin and cytokcratin compared with fixed frozen material. The authors conclude that these cytoskclctal stains can differentiate human Irabeciilar-meshwork cells from cells of the cornea in situ. -

Decreased Expression of Profilin 2 in Oral Squamous Cell Carcinoma and Its Clinicopathological Implications
ONCOLOGY REPORTS 26: 813-823, 2011 Decreased expression of profilin 2 in oral squamous cell carcinoma and its clinicopathological implications C.Y. MA1,2, C.P. ZHANG1,2, L.P. ZHONG1,2, H.Y. PAN1,2, W.T. CHEN1,2, L.Z. WANG3, O.W. ANDREW4, T. JI1 and W. HAN1,2 1Department of Oral and Maxillofacial Surgery, Ninth People's Hospital, College of Stomatology; 2Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology; 3Department of Oral Pathology, Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, P.R. China; 4Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, National University of Singapore, Singapore 119074, Singapore Received February 8, 2011; Accepted April 11, 2011 DOI: 10.3892/or.2011.1365 Abstract. Profilins are small proteins essential for many clinical and pathological significance. In conclusion, PFN2 normal cellular dynamics and constitute one of the crucial can be utilized as both a potential suppressor marker and a components of actin-based cellular motility. Several recent prognostic protein for OSCC. The function of PFN2 may be to studies have implicated a role for the profilin (PFN) family in regulate the N-WASP/Arp2/3 signaling pathway. cancer pathogenesis and progression. However, their expression and promising functions are largely unknown in oral squamous Introduction cell carcinoma (OSCC). In this study, we analyzed the correlation between PFN1 and PFN2 expression in vitro and Oral squamous cell carcinoma (OSCC) is a significant public in vivo. The protein expression levels were roughly compared health problem with >300,000 new cases being diagnosed between cell lines (HIOEC, HB96) with the employment of annually worldwide (1). -

A Significant Soluble Keratin Fraction In
Journal of Cell Science 105, 433-444 (1993) 433 Printed in Great Britain © The Company of Biologists Limited 1993 A significant soluble keratin fraction in ‘simple’ epithelial cells Lack of an apparent phosphorylation and glycosylation role in keratin solubility Chih-Fong Chou*, Carrie L. Riopel, Lusijah S. Rott and M. Bishr Omary† Palo Alto Veterans Administration Medical Center and the Digestive Disease Center at Stanford University, School of Medicine, 3801 Miranda Avenue, GI 111, Palo Alto, CA 94304, USA *Author for reprint requests †Author for correspondence SUMMARY We studied the solubility of keratin polypeptides 8 and aments in vitro as determined by electron microscopy. 18 (K8/18), which are the predominant intermediate fil- Cross-linking of soluble K8/18 followed by immunopre- aments in the human colonic epithelial cell line HT29. cipitation resulted in dimeric and tetrameric forms, We find that asynchronously growing cells (G0/G1 stage based on migration in SDS-polyacrylamide gels. In of the cell cycle) have a substantial pool of soluble ker- addition, cross-linked and native soluble K8/18 showed atin that constitutes approx. 5% of total cellular ker- similar migration on nondenaturing gels and similar atin. This soluble keratin pool was observed after sedimentation after sucrose density gradient centrifu- immunoprecipitation of K8/18 from the cytosolic frac- gation. Our results indicate that simple epithelial ker- tion of cells disrupted using three detergent-free meth- atins are appreciably more soluble than previously rec- ods. Several other cell lines showed a similar significant ognized. The soluble keratin form is assembly competent soluble cytosolic K8/18 pool. -
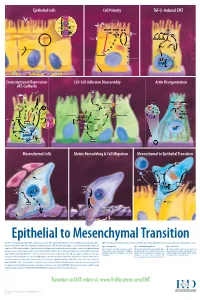
Epithelial to Mesenchymal Transition
Epithelial Cells Cell Polarity TGF-b-Induced EMT MUC-1 O-glycosylation Epithelial Cells ZO-1 Occludin Apical Membrane Tight F-Actin Microvilli Junction Claudin F-Actin p120 β-Catenin Adherens F-Actin Ezrin TGF-β dimer Junction E-Cadherin α-Catenin Plakophilin Crumbs Complex PAR Complex Desmocollin Desmoplakin Desmosome PtdIns(4,5)P2 TGF-β RII TGF-β RI CRB Cdc42Par6 Desmoglein Cytokeratin Pals1 PatJ Tight Junction Plakoglobin aPKC Par3 Domain Smad7 Extracellular PTEN JNK ERK1/2 p38 SARA Smurf1 Cortical Actin Cytoskeleton Space Par3 ZO-1 Adherens Junction PI 3-K Domain Smad-independent Signaling (–) Smad7 Translocation Smad2/3 PtdIns(3,4,5)P3 Smad4 Smad4 NEDD4 Cytokeratin Intermediate Filaments Smad2 Smad4 Smad3 LLGL Proteasome SCRIB DLG Scribble Complex Fibronectin Twist Smad2/3 Vitronectin ZEB 1/2 Microtubule Network Smad4 N-Cadherin Snail Basolateral Membrane CoA, Collagen I Slug CoR MMPs DNA-binding (+) Claudin Desmoplakin Transcription Factor Occludin Cytokeratins E-Cadherin Plakoglobin Integrins β α Nidogen-1/Entactin Perlecan Laminin Collagen IV Transcriptional Repression Cell-Cell Adhesion Disassembly Actin Reorganization of E-Cadherin TGF-β dimer EGF TGF-β RII TGF-β RI IGF FGF Receptor TNF-α Tyrosine Kinase Par6 TNF RI Apical Focal Adhesion Constriction Actin Depolymerization F-Actin Smurf1 Occludin Wnt Frizzled Myosin II Ras RhoA α-Actinin Myosin II ROCK AxinCK1 Dishevelled GSK-3 PI 3-K Src Zyxin MLC Phosphatase APC Proteasome FAK Vinculin RhoA ILK Talin (Inactive) Hakai Talin FAK F-Actin E-Cadherin LIMK Akt Paxillin FAK Stress -

Structures of the ß-Keratin Filaments and Keratin Intermediate Filaments in the Epidermal Appendages of Birds and Reptiles (Sauropsids)
G C A T T A C G G C A T genes Review Structures of the ß-Keratin Filaments and Keratin Intermediate Filaments in the Epidermal Appendages of Birds and Reptiles (Sauropsids) David A.D. Parry School of Fundamental Sciences, Massey University, Private Bag 11-222, Palmerston North 4442, New Zealand; [email protected]; Tel.: +64-6-9517620; Fax: +64-6-3557953 Abstract: The epidermal appendages of birds and reptiles (the sauropsids) include claws, scales, and feathers. Each has specialized physical properties that facilitate movement, thermal insulation, defence mechanisms, and/or the catching of prey. The mechanical attributes of each of these appendages originate from its fibril-matrix texture, where the two filamentous structures present, i.e., the corneous ß-proteins (CBP or ß-keratins) that form 3.4 nm diameter filaments and the α-fibrous molecules that form the 7–10 nm diameter keratin intermediate filaments (KIF), provide much of the required tensile properties. The matrix, which is composed of the terminal domains of the KIF molecules and the proteins of the epidermal differentiation complex (EDC) (and which include the terminal domains of the CBP), provides the appendages, with their ability to resist compression and torsion. Only by knowing the detailed structures of the individual components and the manner in which they interact with one another will a full understanding be gained of the physical properties of the tissues as a whole. Towards that end, newly-derived aspects of the detailed conformations of the two filamentous structures will be discussed and then placed in the context of former knowledge. -

Contralateral Recurrence of Aggressive Fibromatosis in a Young Woman: a Case Report and Review of the Literature
ONCOLOGY LETTERS 10: 325-328, 2015 Contralateral recurrence of aggressive fibromatosis in a young woman: A case report and review of the literature CHRISTOPHER J. SCHMOYER, HARMAR D. BRERETON and ERIC W. BLOMAIN Clinical Faculty, Department of Medicine, The Commonwealth Medical College, Scranton, PA 18509, USA Received August 9, 2014; Accepted April 24, 2015 DOI: 10.3892/ol.2015.3215 Abstract. Aggressive fibromatosis (AF) is a benign and shoulder girdle. Individuals with familial adenomatous non-encapsulated tumor of mesenchymal origin, with a polyposis (FAP) or Gardner's syndrome have a 1,000 times tendency for local spread along fascial planes. Local inva- greater risk for developing the disease due to inheritance of sion can lead to extensive morbidity and even mortality due the adenomatous polyposis coli (APC) gene (3). These patients to destruction of the bones, organs and soft tissues. This rare may present with intra-abdominal lesions following colonic lesion is observed 1,000 times more frequently in patients with resection (4). While AF does not metastasize, local recurrence familial adenomatous polyposis or Gardner's syndrome due to is common. Distant recurrence is extremely rare, but is typi- the inheritance of the adenomatous polyposis coli (APC) gene. cally observed in those with a new primary tumor associated While AF does not metastasize, local recurrence is common. with the APC mutation. The present study reports the case of Distant recurrence is extremely rare, but is observed in those a 20-year-old female with sporadic contralateral recurrence of with a germ line APC mutation. The present study details clinically diagnosed AF and no familial predisposition. -

Differential Organization of Desmin and Vimentin in Muscle Is Due to Differences in Their Head Domains Robert B
Differential Organization of Desmin and Vimentin in Muscle Is Due to Differences in Their Head Domains Robert B. Cary and Michael W. Klymkowsky Molecular, Cellular and Developmental Biology, University of Colorado, Boulder, Colorado 80309-0347 Abstract. In most myogenic systems, synthesis of the aggregates. In embryonic epithelial cells, both vimen- intermediate filament (IF) protein vimentin precedes tin and desmin formed extended IF networks. Vimen- the synthesis of the muscle-specific IF protein desmin. tin and desmin differ most dramatically in their NH:- In the dorsal myotome of the Xenopus embryo, how- terminal "head" regions. To determine whether the ever, there is no preexisting vimentin filament system head region was responsible for the differences in the and desmin's initial organization is quite different from behavior of these two proteins, we constructed plas- that seen in vimentin-containing myocytes (Cary and mids encoding chimeric proteins in which the head of Klymkowsky, 1994. Differentiation. In press.). To de- one was attached to the body of the other. In muscle, termine whether the organization of IFs in the Xeno- the vimentin head-desmin body (VDD) polypeptide pus myotome reflects features unique to Xenopus or is formed longitudinal IFs and massive IF bundles like due to specific properties of desmin, we used the in- vimentin. The desmin head-vimentin body (DVV) jection of plasmid DNA to drive the synthesis of polypeptide, on the other hand, formed IF meshworks vimentin or desmin in myotomal cells. At low levels and non-filamentous structures like desmin. In em- of accumulation, exogenous vimentin and desmin both bryonic epithelial cells DVV formed a discrete fila- enter into the endogenous desmin system of the myo- ment network while VDD did not. -

Identification of Previously Unrecognized FAP in Children With
European Journal of Human Genetics (2015) 23, 715–718 & 2015 Macmillan Publishers Limited All rights reserved 1018-4813/15 www.nature.com/ejhg SHORT REPORT Identification of previously unrecognized FAP in children with Gardner fibroma Joana Vieira1,5, Carla Pinto1,5, Mariana Afonso2,5, Maria do Bom Sucesso3, Paula Lopes2, Manuela Pinheiro1, Isabel Veiga1, Rui Henrique2,4 and Manuel R Teixeira*,1,4 Fibromatous soft tissue lesions, namely desmoid-type fibromatosis and Gardner fibroma, may occur sporadically or as a result of inherited predisposition (as part of familial adenomatous polyposis, FAP). Whereas desmoid-type fibromatosis often present b-catenin overexpression (by activating CTNNB1 somatic variants or APC biallelic inactivation), the pathogenetic mechanisms in Gardner fibroma are unknown. We characterized in detail Gardner fibromas diagnosed in two infants to evaluate their role as sentinel lesions of previously unrecognized FAP. In the first infant we found a 5q deletion including APC in the tumor and the novel APC variant c.4687dup in constitutional DNA. In the second infant we found the c.5826_5829del and c.1678A4T APC variants in constitutional and tumor DNA, respectively. None of the constitutional APC variants occurred de novo and both tumors showed nuclear staining for b-catenin and no CTNNB1 variants. We present the first comprehensive characterization of the pathogenetic mechanisms of Gardner fibroma, which may be a sentinel lesion of previously unrecognized FAP families. European Journal of Human Genetics (2015) 23, 715–718; doi:10.1038/ejhg.2014.144; published online 30 July 2014 INTRODUCTION MATERIALS AND METHODS Familial adenomatous polyposis (FAP) is an autosomal dominant The first case was a 5-month-old child who had two lumbar subcutaneous disease caused by APC constitutional variants. -

Damage of Hair Follicle Stem Cells and Alteration of Keratin Expression in External Radiation-Induced Acute Alopecia
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 30: 579-584, 2012 Damage of hair follicle stem cells and alteration of keratin expression in external radiation-induced acute alopecia NAOKI NANASHIMA, KOICHI ITO, TAKASHI ISHIKAWA, MANABU NAKANO and TOSHIYA NAKAMURA Department of Biomedical Sciences, Division of Medical Life Sciences, Hirosaki University Graduate School of Health Sciences, Hirosaki, Japan Received April 4, 2012; Accepted May 28, 2012 DOI: 10.3892/ijmm.2012.1018 Abstract. Alopecia is known as a symptom of acute radia- disturbances and blood and bone marrow disorders are known tion, yet little is known concerning the mechanism of this to occur within several hours to several weeks after 1-6 Gy of phenomenon and the alteration of hair protein profiles. To radiation exposure (4,6). examine this, 6-week-old male C57/BL6 mice were exposed Hair loss is also an effect of ARS, but little is known to 6 Gy of X-ray irradiation, which caused acute alopecia. about the mechanism underlying radiation-induced hair loss. Their hair and skin were collected, and hair proteins were In humans, hair loss is caused by radiation of more than analyzed with liquid chromatography/electrospray-ionization 3 Gy, and almost complete hair loss occurs within weeks of mass spectrometry and immunohistochemistry. No change exposure to 6 Gy (4,6). Since blood stem cells are sensitive to was observed in the composition of major hair keratins, such radiation (7), hair loss is thought to be caused by irradiation- as Krt81, Krt83 and Krt86. However, cytokeratin Krt15 and induced stem cell damage, yet no studies have investigated CD34, which are known as hair follicle stem cell markers, this hypothesis. -

Disease-Proportional Proteasomal Degradation of Missense Dystrophins
Disease-proportional proteasomal degradation of missense dystrophins Dana M. Talsness, Joseph J. Belanto, and James M. Ervasti1 Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota–Twin Cities, Minneapolis, MN 55455 Edited by Louis M. Kunkel, Children’s Hospital Boston, Harvard Medical School, Boston, MA, and approved September 1, 2015 (received for review May 5, 2015) The 427-kDa protein dystrophin is expressed in striated muscle insertions or deletions (indels) represent ∼7% of the total DMD/ where it physically links the interior of muscle fibers to the BMD population (13). When indel mutations cause a frameshift, they extracellular matrix. A range of mutations in the DMD gene encod- can specifically be targeted by current exon-skipping strategies (15). ing dystrophin lead to a severe muscular dystrophy known as Du- Patients with missense mutations account for only a small percentage chenne (DMD) or a typically milder form known as Becker (BMD). of dystrophinopathies (<1%) (13), yet they represent an orphaned Patients with nonsense mutations in dystrophin are specifically tar- subpopulation with an undetermined pathomechanism and no cur- geted by stop codon read-through drugs, whereas out-of-frame de- rent personalized therapies. letions and insertions are targeted by exon-skipping therapies. Both The first missense mutation reported to cause DMD was L54R treatment strategies are currently in clinical trials. Dystrophin mis- in ABD1 of an 8-y-old patient (16). Another group later reported sense mutations, however, cause a wide range of phenotypic se- L172H, a missense mutation in a structurally analogous location of verity in patients. The molecular and cellular consequences of such ABD1 (17), yet this patient presented with mild symptoms at 42 mutations are not well understood, and there are no therapies spe- years of age. -

A Dysfunctional Desmin Mutation in a Patient with Severe Generalized Myopathy
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 11312–11317, September 1998 Genetics A dysfunctional desmin mutation in a patient with severe generalized myopathy ANA M. MUN˜OZ-MA´RMOL*†‡,GERALDINE STRASSER‡§,MARCOS ISAMAT*†,PIERRE A. COULOMBE¶,YANMIN YANG§, i XAVIER ROCA†,ELENA VELA*†,JOSE´ L. MATE†,JAUME COLL ,MARI´A TERESA FERNA´NDEZ-FIGUERAS†, JOSE´ J. NAVAS-PALACIOS†,AURELIO ARIZA†, AND ELAINE FUCHS§** *Fundacio´n Echevarne, 08037 Barcelona, Spain; Departments of †Pathology and iNeurology, Hospital Universitari Germans Trias i Pujol, Universitat Auto´noma de Barcelona, 08916 Badalona, Barcelona, Spain; ¶Department of Biological Chemistry, The Johns Hopkins University School of Medicine, Baltimore, MD, 21205; and §Howard Hughes Medical Institute and Department of Molecular Genetics and Cell Biology, The University of Chicago, Chicago, IL 60637 Contributed by Elaine Fuchs, July 29, 1998 ABSTRACT Mice lacking desmin produce muscle fibers desmin functions in cellular transmission of both active and with Z disks and normal sarcomeric organization. However, passive force (14, 15). the muscles are mechanically fragile and degenerate upon Desminopathies are a heterogeneous group of human myop- repeated contractions. We report here a human patient with athies characterized by abnormal aggregations of desmin in severe generalized myopathy and aberrant intrasarcoplasmic skeletal andyor cardiac muscle fibers (for review, see ref. 16). The accumulation of desmin intermediate filaments. Muscle tissue clumping of desmin in muscle cells of these patients bears a from this patient lacks the wild-type desmin allele and has a striking resemblance to the keratin aggregates that accumulate in desmin gene mutation encoding a 7-aa deletion within the degenerating epithelial cells of various human keratin disorders coiled-coil segment of the protein.