In Situ Leach (ISL) Mining of Uranium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

About Uranium Mining in South Australia Foreword
The Facts about uranium mining in South Australia Foreword South Australia has been a major producer of uranium since 1988. We are proud of our track record and our global reputation for excellence. The South Australian Government thoroughly To achieve that aim we need to challenge assess mining lease proposals, and through the perceptions of unacceptable hazards stringent conditions, rigorously upholds the associated with the uranium industry. Risks highest standards for monitoring and safety. associated with nuclear energy are judged harsher than competing energy sources. The enduring strength of this State’s leadership in uranium mining is an insistence Access to information and education is on world’s best practice for managing our the key to challenging these perceptions. resources. Uranium – The Facts is just that, the facts that should be the basis for any informed debate Our global reputation enables us to attract about uranium and South Australia’s current the world’s leading uranium miners and role in the global nuclear fuel cycle. lead the country in annual production. Uranium produced in South Australia is equivalent to delivering CO2-free power to 20 million people. Yet with more than 80% of Australia’s total Hon Tom Koutsantonis MP, uranium resource, there remains considerable Minister for Mineral Resources and Energy scope to expand. It’s not enough that we produce exports from the world’s largest uranium deposit at Olympic Dam, we want to unlock the full potential of all South Australia’s uranium assets. How we regulate The Foreign Investment Review The Australian regulatory framework Board examines foreign investment for the uranium industry is widely proposals to ensure the investment is recognised as world’s best practice. -

Understanding Fluid–Rock Interactions and Lixiviant/Oxidant Behaviour for the In-Situ Recovery of Metals from Deep Ore Bodies
School of Earth and Planetary Science Department of Applied Geology Understanding Fluid–Rock Interactions and Lixiviant/Oxidant Behaviour for the In-situ Recovery of Metals from Deep Ore Bodies Tania Marcela Hidalgo Rosero This thesis is presented for the degree of Doctor of Philosophy of Curtin University February 2020 1 Declaration __________________________________________________________________________ Declaration To the best of my knowledge and belief, I declare that this work of thesis contains no material published by any other person, except where due acknowledgements have been made. This thesis contains no material which has been accepted for the award of any other degree or diploma in any university. Tania Marcela Hidalgo Rosero Date: 28/01/2020 2 Abstract __________________________________________________________________________ Abstract In-situ recovery (ISR) processing has been recognised as a possible alternative to open- pit mining, especially for low-grade resources. In ISR, the fluid–rock interaction between the target ore and the lixiviant results in valuable- (and gangue-) metal dissolution. This interaction is achieved by the injection and recovery of fluid by means of strategically positioned wells. Although the application of ISR has become more common (ISR remains the preferential processing technique for uranium and has been applied in pilot programs for treating oxide zones in copper deposits), its application to hard-rock refractory and low-grade copper-sulfide deposits is still under development. This research is focused on the possible application of ISR to primary copper sulfides usually found as deep ores. Lixiviant/oxidant selection is an important aspect to consider during planning and operation in the ISR of copper-sulfide ores. -

Arkaroola Protection Area: a Field Guide to Selected Geological Features
Arkaroola Protection Area: A field guide to selected geological features Graeme L. Worboys and Stephen B. Hore arkaroola.com.au environment.sa.gov.au Citation: Worboys, G. L. and Hore, S.B. (2013) Arkaroola Protection Area: A field guide to selected geological features. Arkaroola Wilderness Sanctuary and Department of Environment, Water and Natural Resources, Adelaide. Copyright: © This work is copyright. Apart from any use permitted under the Australian Copyright Act 1968, no part may be reproduced by any process, nor may any other exclusive right be exercised without the express permission of the authors. Acknowledgements: Many individuals and organisations contributed to the development of this Field Guide. The text has been sourced predominantly from the Arkaroola National Heritage Listing nomination jointly submitted to the Australian Government by the South Australian Department of Environment, Water and Natural Resources and Margaret and Douglas Sprigg of the Arkaroola Wilderness Sanctuary. Appreciation is expressed for the use of this material. The Field Guide also sourced technical geological quotes from a 2004 field guide developed by John Drexel and Stephen Hore and appreciation is extended for the use of this material. Thanks are particularly extended to Margaret and Douglas Sprigg, Lorraine Edmunds and Dennis Walter of Arkaroola Wilderness Sanctuary; Jason Irving of the South Australian Department of Environment, Water and Natural Resources; Tim Baker of the Geological Survey of South Australia; the Geological Society of Australia (South Australia Division); Jim Gehling and Joël Brugger of the South Australian Museum; the University of Adelaide; Malcolm William Wallace of the University of Melbourne; Malcolm Walter of the University of New South Wales; Narelle Neumann of Geoscience Australia; and Paul O’Brien of Helivista Helicopters (South Australia) for their assistance in the development of this material. -
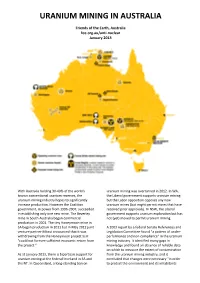
4 Pages on U Mining by End of October, See Pol D
URANIUM MINING IN AUSTRALIA Friends of the Earth, Australia foe.org.au/anti-nuclear January 2013 With Australia holding 30-40% of the world's uranium mining was overturned in 2012. In WA, known conventional uranium reserves, the the Liberal government supports uranium mining uranium mining industry hopes to significantly but the Labor opposition opposes any new increase production. However the Coalition uranium mines (but might permit mines that have government, in power from 1996-2007, succeeded received prior approvals). In NSW, the Liberal in establishing only one new mine. The Beverley government supports uranium exploration but has mine in South Australia began commercial not (yet) moved to permit uranium mining. production in 2001. The tiny Honeymoon mine in SA began production in 2011 but in May 2012 joint A 2003 report by a federal Senate References and venture partner Mitsui announced that it was Legislation Committee found "a pattern of under- withdrawing from the Honeymoon project as it performance and non-compliance" in the uranium "could not foresee sufficient economic return from mining industry. It identified many gaps in the project." knowledge and found an absence of reliable data on which to measure the extent of contamination As at January 2013, there is bipartisan support for from the uranium mining industry, and it uranium mining at the federal level and in SA and concluded that changes were necessary "in order the NT. In Queensland, a long-standing ban on to protect the environment and its inhabitants from serious or irreversible damage". The history of secret nuclear weapons research, and committee concluded "that short-term states stockpiling 'civil' plutonium. -

Precipitation of Aluminum Containing Species in Tank Wastes
PNNL-13881 Precipitation of Aluminum Containing Species in Tank Wastes S.V. Mattigod K.E. Parker D.T. Hobbs D.E. McCready April 2002 Prepared for the U.S. Department of Energy under Contract DE-AC06-76RL01830 PNNL-13881 DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor Battelle Memorial Institute, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or Battelle Memorial Institute. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. PACIFIC NORTHWEST NATIONAL LABORATORY operated by BATTELLE for the UNITED STATES DEPARTMENT OF ENERGY under Contract DE-AC06-76RL01830 This document was printed on recycled paper. (8/00 PNNL-13881 Precipitation of Aluminum Containing Species in Tank Wastes S. V. Mattigod D. T. Hobbs K. E. Parker D. E. McCready April 2002 Prepared for the U.S. Department of Energy under Contract DE-AC06-76RL01830 Pacific Northwest National Laboratory Richland, Washington 99352 Summary Aluminisilicate deposit buildup experienced during the tank waste volume-reduction process at the Savannah River Site (SRS) required an evaporator to be shut down in October 1999. -

Sell-1559, Leaching
CONTACT INFORMATION Mining Records Curator Arizona Geological Survey 416 W. Congress St., Suite 100 Tucson, Arizona 85701 520-770-3500 http://www.azgs.az.gov [email protected] The following file is part of the James Doyle Sell Mining Collection ACCESS STATEMENT These digitized collections are accessible for purposes of education and research. We have indicated what we know about copyright and rights of privacy, publicity, or trademark. Due to the nature of archival collections, we are not always able to identify this information. We are eager to hear from any rights owners, so that we may obtain accurate information. Upon request, we will remove material from public view while we address a rights issue. CONSTRAINTS STATEMENT The Arizona Geological Survey does not claim to control all rights for all materials in its collection. These rights include, but are not limited to: copyright, privacy rights, and cultural protection rights. The User hereby assumes all responsibility for obtaining any rights to use the material in excess of “fair use.” The Survey makes no intellectual property claims to the products created by individual authors in the manuscript collections, except when the author deeded those rights to the Survey or when those authors were employed by the State of Arizona and created intellectual products as a function of their official duties. The Survey does maintain property rights to the physical and digital representations of the works. QUALITY STATEMENT The Arizona Geological Survey is not responsible for the accuracy of the records, information, or opinions that may be contained in the files. The Survey collects, catalogs, and archives data on mineral properties regardless of its views of the veracity or accuracy of those data. -

Comparative Study on Precipitation Methods of Yellow
Precipitation and purification of uranium from rock phosphate Elshafeea H. Y. Abow Slama1, Etemad Ebraheem2 and Adam K. Sam1 1Sudan Atomic Energy Commission P.O. Box 3001, Khartoum-Sudan 2 Sinner University ABSTRACT This study was carried-out to leach uranium from rock phosphate using sulphuric acid in the presence of potassium chlorate as an oxidant and to investigate the relative purity of different forms of yellow cakes produced with ammonia {( NH 4 )2 U2O7 }, magnesia (UO3.xH 2O ) and sodium hydroxide Na2U 2O7 as precipitants, as well as purification of the products with TBP extraction and matching its impurity levels with specifications of the commercial products. Alpha-particle spectrometry was used for determination of activity concentration of uranium isotopes (234U and 238U) in rock phosphate, resulting green phosphoric acid solution, and in different forms of the yellow cake from which the equivalent mass concentration of uranium was deduced. Likewise, atomic absorption spectroscopy (AAS) was used for determination of impurities (Pb, Ni, Cd, Fe, Zn, Mn, and Cu). On the average, the equivalent mass concentration of uranium was 119.38±79.66 ppm (rock phosphate) and 57.85±20.46 ppm (green solution) with corresponding low percent of dissolution (48%) which is considered low. The isotopic ratio (234U: 238U) in all stages of hydrometallurgical process was not much differ from unity indicating lack of fractionation. Upon comparing the levels of impurities in different form of crude yellow cakes, it was found that the lowest levels were measured in UO3.xH2O. This implies that saturated magnesia is least aggressive relative to other precipitants and gives relatively pure crude cake. -

Uranium, Cesium, and Mercury Leaching and Recovery from Cemented Radioactive Wastes in Sulfuric Acid and Iodide Media
Article Uranium, Cesium, and Mercury Leaching and Recovery from Cemented Radioactive Wastes in Sulfuric Acid and Iodide Media Nicolas Reynier 1,*, Rolando Lastra 1, Cheryl Laviolette 1, Jean-François Fiset 1, Nabil Bouzoubaâ 1 and Mark Chapman 2 Received: 28 September 2015 ; Accepted: 10 November 2015 ; Published: 20 November 2015 Academic Editor: Mostafa Fayek 1 CanmetMINING, Natural Resources Canada, 3484 Limebank Rd., Ottawa, ON K1A 0E4, Canada; [email protected] (R.L.); [email protected] (C.L.); jean-francois.fi[email protected] (J.-F.F.); [email protected] (N.B.) 2 Canadian Nuclear Laboratories, Chalk River Laboratories, Plant Road, Chalk River, ON K0J 1 J0, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-613-954-5602; Fax: +1-613-954-6929 Abstract: The Canadian Nuclear Laboratories (CNL) is developing a long-term management strategy for its existing inventory of solid radioactive cemented wastes, which contain uranium, mercury, fission products, and a number of minor elements. The composition of the cemented radioactive waste poses significant impediments to the extraction and recovery of uranium using conventional technology. The goal of this research was to develop an innovative method for uranium, mercury and cesium recovery from surrogate radioactive cemented waste (SRCW). Leaching using sulfuric acid and saline media significantly improves the solubilization of the key elements from the SRCW. Increasing the NaCl concentration from 0.5 to 4 M increases the mercury solubilization from 82% to 96%. The sodium chloride forms a soluble mercury complex when mercury is present as HgO or metallic mercury but not with HgS that is found in 60 ˝C cured SRCW. -

Uranium Mining and Nuclear Facilities (Prohibitions) Repeal Bill 2019
LEGISLATIVE COUNCIL STANDING COMMITTEE ON STATE DEVELOPMENT Uranium Mining and Nuclear Facilities (Prohibitions) Repeal Bill 2019 Report 46 March 2020 www.parliament.nsw.gov.au LEGISLATIVE COUNCIL Standing Committee on State Development Uranium Mining and Nuclear Facilities (Prohibitions) Repeal Bill 2019 Ordered to be printed 4 March 2020 according to Standing Order 231 Report 46 - March 2020 i LEGISLATIVE COUNCIL Uranium Mining and Nuclear Facilities (Prohibitions) Repeal Bill 2019 New South Wales Parliamentary Library cataloguing-in-publication data: New South Wales. Parliament. Legislative Council. Standing Committee on State Development. Uranium Mining and Nuclear Facilities (Prohibitions) Repeal Bill 2019 / Standing Committee on State Development. [Sydney, N.S.W.] : the Committee, 2020. – [xiv, 150] pages ; 30 cm. (Report no. 46 / Standing Committee on State Development) Chair: Hon. Taylor Martin, MLC. “March 2020” ISBN 9781920788599 1. New South Wales. Parliament. Legislative Council—Uranium Mining and Nuclear Facilities (Prohibitions) Repeal Bill 2018. 2. Uranium mines and mining—Law and legislation—New South Wales. 3. Nuclear industry—Law and legislation—New South Wales. 4. Nuclear energy—Law and legislation—New South Wales. I. Martin, Taylor. II. Title. III. Series: New South Wales. Parliament. Legislative Council. Standing Committee on State Development. Report ; no. 46 622.349 (DDC22) ii Report 46 - March 2020 STANDING COMMITTEE ON STATE DEVELOPMENT Table of contents Terms of reference vi Committee details vii Chair’s foreword -

Modification of Base-Side 99Mo Production Processes for Leu Metal-Foil Targets
MODIFICATION OF BASE-SIDE 99MO PRODUCTION PROCESSES FOR LEU METAL-FOIL TARGETS G. F. Vandegrift, R. A. Leonard, S. Aase, J. Sedlet, Y. Koma, C. Conner, C. R. Clark, and M. K. Meyer Argonne National Laboratory Argonne, Illinois, and Idaho Falls, Idaho, U.S.A. ABSTRACT Argonne National Laboratory is cooperating with the National Atomic Energy Commission of the Argentine Republic (CNEA) to convert their 99Mo production process, which uses high enriched uranium (HEU), to low-enriched uranium (LEU). The program is multifaceted; however, discussed in this paper are (1) results of laboratory experiments to develop means for substituting LEU metal-foil targets into the current process and (2) preparation of uranium-alloy or uranium-metal/aluminum-dispersion targets. Although 99Mo production is a multi-step process, the first two steps (target dissolution and primary molybdenum recovery) are by far the most important in the conversion. Commonly, once molybdenum is separated from the bulk of the uranium, the remainder of the process need not be modified. Our results show that, up to this point in our study, conversion of the CNEA process to LEU appears viable. INTRODUCTION A number of current producers dissolve uranium-aluminide/aluminum-dispersion plates in alkaline solution as an initial step to recovering fission-product 99Mo from irradiated high- enriched uranium (HEU). These producers include Argentine Comisión Nacional de Energía Atómica (CNEA), Institut National des Radioéléments (IRE), Mallinckrodt, and the Atomic Energy Corporation of South Africa Limited (AEC). Argonne National Laboratory (ANL) has begun a cooperation with one of these producers, CNEA, to convert their process to low- enriched uranium (LEU). -

Governing Uranium in Australia
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Vestergaard, Cindy Research Report Governing uranium in Australia DIIS Report, No. 2015:11 Provided in Cooperation with: Danish Institute for International Studies (DIIS), Copenhagen Suggested Citation: Vestergaard, Cindy (2015) : Governing uranium in Australia, DIIS Report, No. 2015:11, ISBN 978-87-7605-762-6, Danish Institute for International Studies (DIIS), Copenhagen This Version is available at: http://hdl.handle.net/10419/144725 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. -

Generic Environmental Impact Statement for In-Situ
GENERIC ENVIRONMENTAL IMPACT STATEMENT FOR IN-SITU LEACH URANIUM MILLING FACILITIES SCOPING SUMMARY REPORT JUNE 2008 U.S. Nuclear Regulatory Commission Rockville, Maryland 1. INTRODUCTION The U.S. Nuclear Regulatory Commission (NRC) expects to receive a number of new license applications for uranium milling at sites in the states of Nebraska, South Dakota, Wyoming and New Mexico over the next several years. NRC anticipates that most of these potential license applications will involve uranium milling facilities that would use the in-situ leach (ISL) process. Because there are environmental issues common to ISL milling facilities, NRC has prepared a Generic Environmental Impact Statement (GEIS) to evaluate the potential environmental impacts associated with the construction, operation, aquifer restoration, and decommissioning at future ISL milling facilities in specific regions of interest within these four western states, where NRC is the licensing authority for uranium milling. In the ISL process, a leaching agent, such as oxygen with sodium bicarbonate, is added to native ground water for injection through wells into the subsurface ore body to dissolve the uranium. The leach solution, containing the dissolved uranium, is pumped back to the surface and sent to a processing plant, where ion exchange is used to separate the uranium from the solution. The underground leaching of the uranium also frees other metals and minerals from the host rock. Operators of ISL facilities are required to restore the ground water affected by the leaching operations. The milling process concentrates the recovered uranium into the product known as "yellowcake" (U3O8). This yellowcake is then shipped to uranium conversion facilities for further processing in the overall uranium fuel cycle.