A Perennial Flax (Linum Spp.) Breeding Program Using Ideotype Models to Select for Oilseed, Garden, and Cut Flower Cultivars
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of Isoprenyl Diphosphate Synthase-Like Terpene
www.nature.com/scientificreports OPEN Evolution of isoprenyl diphosphate synthase‑like terpene synthases in fungi Guo Wei1, Franziska Eberl2, Xinlu Chen1, Chi Zhang1, Sybille B. Unsicker2, Tobias G. Köllner2, Jonathan Gershenzon2 & Feng Chen1* Terpene synthases (TPSs) and trans‑isoprenyl diphosphate synthases (IDSs) are among the core enzymes for creating the enormous diversity of terpenoids. Despite having no sequence homology, TPSs and IDSs share a conserved “α terpenoid synthase fold” and a trinuclear metal cluster for catalysis, implying a common ancestry with TPSs hypothesized to evolve from IDSs anciently. Here we report on the identifcation and functional characterization of novel IDS-like TPSs (ILTPSs) in fungi that evolved from IDS relatively recently, indicating recurrent evolution of TPSs from IDSs. Through large-scale bioinformatic analyses of fungal IDSs, putative ILTPSs that belong to the geranylgeranyl diphosphate synthase (GGDPS) family of IDSs were identifed in three species of Melampsora. Among the GGDPS family of the two Melampsora species experimentally characterized, one enzyme was verifed to be bona fde GGDPS and all others were demonstrated to function as TPSs. Melampsora ILTPSs displayed kinetic parameters similar to those of classic TPSs. Key residues underlying the determination of GGDPS versus ILTPS activity and functional divergence of ILTPSs were identifed. Phylogenetic analysis implies a recent origination of these ILTPSs from a GGDPS progenitor in fungi, after the split of Melampsora from other genera within the class of Pucciniomycetes. For the poplar leaf rust fungus Melampsora larici-populina, the transcripts of its ILTPS genes were detected in infected poplar leaves, suggesting possible involvement of these recently evolved ILTPS genes in the infection process. -

Flax Rust and Its Control
-1 I , r TECHNICAL BULLETIN 36 MARCH 1926 University of Minnesota Agricultural Experiment Station IN CO-OPERATION WITH UNITED STATES DEPARTMENT OF AGRICULTURE, BUREAU OF PLANT INDUSTRY Flax Rust and its Control A. W. Henry Division of Plant Pathology and Botany UNIVERSITY FARM, ST. PAUL AGRICULTURAL EXPERIMENT STATION ADMINISTRATIVE OFFICERS W. C. COFFEY, M.S., Director ANDREW Boss, Vice-Director F. W. PECK, M.S., Director of Agricultural Extension and Farmers' Institutes C. G. SELVIG, M.A., Superintendent, Northwest Substation, Crookston P. E. MILLER, M.Agr., Superintendent, West Central Substation, AIorris 0. I. BERGH, B.S.Agr., Superintendent, North Central Substation, Grand Rapids M. J. THOMPSON, Superintendent, Northeast Substation, Duluth R E. HODGSON, B.S. in Agr., Superintendent, Southeast Substation, Waseca RAPHAEL ZON, F.E., Director, Forest Experiment Station, Cloquet F. E. HARALSON, Assistant Superintendent, Fruit Breeding Farm, Zumbra Heights, (P.O. Excelsior) W. P. KIRKWOOD, M.A., Editor, and Chief, Division of Publications ALICE MCFEELY, Assistant Editor of Bulletins HARRIET W. SEWALL, B.A., Librarian T. J. HORTON, Photographer R. A. GORTNER, Ph.D., Chief, Division of Agricultural Biochemistry J. D. BLACK, Ph.D., Chief, Division of Agricultural Economics WILLIAM Boss, Chief, Division of Agricultural Engineering ANDREW Boss, Chief, Division of Agronomy and Farm Management W. H. PETERS, M.Agr., Chief, Division of Animal Husbandry FRANCIS JAGER, Chief, Division of Bee Culture C. H. EcKLEs, M.S., D.Sc., Chief, Division of Dairy Husbandry R. N. CHAPMAN, Ph.D., Chief Division of Entomology and Economic Zoology HENRY ScH/Arrz, Ph.D., Chief, Division of Forestry W. H. ALDERMAN, B.S.A., Chief, Division of Horticulture E. -

FLORA from FĂRĂGĂU AREA (MUREŞ COUNTY) AS POTENTIAL SOURCE of MEDICINAL PLANTS Silvia OROIAN1*, Mihaela SĂMĂRGHIŢAN2
ISSN: 2601 – 6141, ISSN-L: 2601 – 6141 Acta Biologica Marisiensis 2018, 1(1): 60-70 ORIGINAL PAPER FLORA FROM FĂRĂGĂU AREA (MUREŞ COUNTY) AS POTENTIAL SOURCE OF MEDICINAL PLANTS Silvia OROIAN1*, Mihaela SĂMĂRGHIŢAN2 1Department of Pharmaceutical Botany, University of Medicine and Pharmacy of Tîrgu Mureş, Romania 2Mureş County Museum, Department of Natural Sciences, Tîrgu Mureş, Romania *Correspondence: Silvia OROIAN [email protected] Received: 2 July 2018; Accepted: 9 July 2018; Published: 15 July 2018 Abstract The aim of this study was to identify a potential source of medicinal plant from Transylvanian Plain. Also, the paper provides information about the hayfields floral richness, a great scientific value for Romania and Europe. The study of the flora was carried out in several stages: 2005-2008, 2013, 2017-2018. In the studied area, 397 taxa were identified, distributed in 82 families with therapeutic potential, represented by 164 medical taxa, 37 of them being in the European Pharmacopoeia 8.5. The study reveals that most plants contain: volatile oils (13.41%), tannins (12.19%), flavonoids (9.75%), mucilages (8.53%) etc. This plants can be used in the treatment of various human disorders: disorders of the digestive system, respiratory system, skin disorders, muscular and skeletal systems, genitourinary system, in gynaecological disorders, cardiovascular, and central nervous sistem disorders. In the study plants protected by law at European and national level were identified: Echium maculatum, Cephalaria radiata, Crambe tataria, Narcissus poeticus ssp. radiiflorus, Salvia nutans, Iris aphylla, Orchis morio, Orchis tridentata, Adonis vernalis, Dictamnus albus, Hammarbya paludosa etc. Keywords: Fărăgău, medicinal plants, human disease, Mureş County 1. -

Structural Genomics Applied to the Rust Fungus Melampsora Larici-Populina
www.nature.com/scientificreports OPEN Structural genomics applied to the rust fungus Melampsora larici- populina reveals two candidate efector proteins adopting cystine knot and NTF2-like protein folds Karine de Guillen1, Cécile Lorrain2, Pascale Tsan3, Philippe Barthe1, Benjamin Petre2, Natalya Saveleva2, Nicolas Rouhier2, Sébastien Duplessis2, André Padilla1 & Arnaud Hecker2* Rust fungi are plant pathogens that secrete an arsenal of efector proteins interfering with plant functions and promoting parasitic infection. Efectors are often species-specifc, evolve rapidly, and display low sequence similarities with known proteins. How rust fungal efectors function in host cells remains elusive, and biochemical and structural approaches have been scarcely used to tackle this question. In this study, we produced recombinant proteins of eleven candidate efectors of the leaf rust fungus Melampsora larici-populina in Escherichia coli. We successfully purifed and solved the three- dimensional structure of two proteins, MLP124266 and MLP124017, using NMR spectroscopy. Although both MLP124266 and MLP124017 show no sequence similarity with known proteins, they exhibit structural similarities to knottins, which are disulfde-rich small proteins characterized by intricate disulfde bridges, and to nuclear transport factor 2-like proteins, which are molecular containers involved in a wide range of functions, respectively. Interestingly, such structural folds have not been reported so far in pathogen efectors, indicating that MLP124266 and MLP124017 may bear novel functions related to pathogenicity. Our fndings show that sequence-unrelated efectors can adopt folds similar to known proteins, and encourage the use of biochemical and structural approaches to functionally characterize efector candidates. To infect their host, flamentous pathogens secrete efector proteins that interfere with plant physiology and immunity to promote parasitic growth1. -

Pollen Flora of Pakistan -Lxi. Violaceae
Pak. J. Bot., 41(1): 1-5, 2009. POLLEN FLORA OF PAKISTAN -LXI. VIOLACEAE ANJUM PERVEEN AND MUHAMMAD QAISER* Department of Botany, University of Karachi, Karachi, Pakistan *Federal Urdu University of Arts, Science and Technology, Karachi, Pakistan. Abstract Pollen morphology of 5 species of the family Violaceae from Pakistan has been examined by light and scanning electron microscope. Pollen grains are usually radially symmetrical, isopolar, colporate, sub-prolate to prolate-spheroidal. Sexine slightly thicker or thinner than nexine. Tectum mostly densely punctate rarely psilate. On the basis of exine pattern two distinct pollen types viz., Viola pilosa–type and Viola stocksii-type are recognized. Introduction Violaceae is a family with 20 genera and about 800 species (Mabberley, 1987). In Pakistan it is represented by one genus and 17 species (Qaiser & Omer, 1985). Plant perennial herbs, or shrubs leaves simple, alternate rarely opposite, flowers bisexual, zygomorphic or actinomorphic, calyx 5, corolla of 5 petals, anterior petal large and spurred. Androecium of 5 stamens. Gynoecium a compound pistil of 3 united carpels, ovules superior, fruit capsule. The family is of little economic importance except for the garden favorite, Violets, Violas and Pansies. Pollen morphology of the family has been examined by Erdtman (1952), Lobreau- Callen (1977), Moore & Webb (1978) and Dojas et al., (1993). Moore et al., (1991) examined pollen morphology of the genus Viola. Kubitzki (2004) examined the pollen morphology of the family Violaceae. There are no reports on pollen morphology of the family Violaceae from Pakistan. Present investigations are based on the pollen morphology of 5 species representing a single genus of the family Violaceae by light and scanning electron microscope. -

Anti-HIV-1 Activities of the Extracts from the Medicinal Plant Linum Grandiflorum Desf
® Medicinal and Aromatic Plant Science and Biotechnology ©2009 Global Science Books Anti-HIV-1 Activities of the Extracts from the Medicinal Plant Linum grandiflorum Desf. Magdy M. D. Mohammed1,2,3* • Lars P. Christensen1,4 • Nabaweya A. Ibrahim3 • Nagwa E. Awad3 • Ibrahim F. Zeid5 • Erik B. Pedersen2 • Kenneth B. Jensen2 • Paolo L. Colla6 1 Danish Institute of Agricultural Sciences (DIAS), Department of Food Science, Research Center Aarslev, Aarhus University, Kirstinebjergvej 10, DK-5792 Aarslev, Denmark 2 Nucleic Acid Center, Institute of Physics and Chemistry, University of Southern Denmark, Campusvej 55, DK-5230 Odense M, Denmark 3 Pharmacognosy Department, National Research Center, Dokki-12311, Cairo, Egypt 4 Institute of Chemical Engineering, Biotechnology and Environmental Technology, University of Southern Denmark, Niels Bohrs Allé 1, DK-5230 Odense M, Denmark 5 Chemistry Department, Faculty of Science, El-Menoufia University, El-Menoufia, Egypt 6 Department of Science and Biomedical Technology, Cittadella University, Monserrato, Italy Corresponding author : * [email protected] ABSTRACT As part of our screening of anti-AIDS agents from natural sources e.g. Ixora undulata, Paulownia tomentosa, Fortunella margarita, Aegle marmelos and Erythrina abyssinica, the different organic and aqueous extracts of Linum grandiflorum leaves and seeds were evaluated in vitro by the microculture tetrazolium (MTT) assay. The activity of the tested extracts against multiplication of HIV-1 wild type IIIB, N119, A17, and EFVR in acutely infected cells was based on inhibition of virus-induced cytopathicity in MT-4 cells. Results revealed that both the MeOH and the CHCl3 extracts of L. grandiflorum have significant inhibitory effects against HIV-1 induced infection with MT-4 cells. -

Checklist of the Vascular Plants of Redwood National Park
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 9-17-2018 Checklist of the Vascular Plants of Redwood National Park James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Checklist of the Vascular Plants of Redwood National Park" (2018). Botanical Studies. 85. https://digitalcommons.humboldt.edu/botany_jps/85 This Flora of Northwest California-Checklists of Local Sites is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A CHECKLIST OF THE VASCULAR PLANTS OF THE REDWOOD NATIONAL & STATE PARKS James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State Univerity Arcata, California 14 September 2018 The Redwood National and State Parks are located in Del Norte and Humboldt counties in coastal northwestern California. The national park was F E R N S established in 1968. In 1994, a cooperative agreement with the California Department of Parks and Recreation added Del Norte Coast, Prairie Creek, Athyriaceae – Lady Fern Family and Jedediah Smith Redwoods state parks to form a single administrative Athyrium filix-femina var. cyclosporum • northwestern lady fern unit. Together they comprise about 133,000 acres (540 km2), including 37 miles of coast line. Almost half of the remaining old growth redwood forests Blechnaceae – Deer Fern Family are protected in these four parks. -
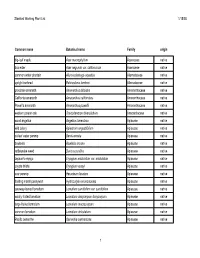
Plant List for Web Page
Stanford Working Plant List 1/15/08 Common name Botanical name Family origin big-leaf maple Acer macrophyllum Aceraceae native box elder Acer negundo var. californicum Aceraceae native common water plantain Alisma plantago-aquatica Alismataceae native upright burhead Echinodorus berteroi Alismataceae native prostrate amaranth Amaranthus blitoides Amaranthaceae native California amaranth Amaranthus californicus Amaranthaceae native Powell's amaranth Amaranthus powellii Amaranthaceae native western poison oak Toxicodendron diversilobum Anacardiaceae native wood angelica Angelica tomentosa Apiaceae native wild celery Apiastrum angustifolium Apiaceae native cutleaf water parsnip Berula erecta Apiaceae native bowlesia Bowlesia incana Apiaceae native rattlesnake weed Daucus pusillus Apiaceae native Jepson's eryngo Eryngium aristulatum var. aristulatum Apiaceae native coyote thistle Eryngium vaseyi Apiaceae native cow parsnip Heracleum lanatum Apiaceae native floating marsh pennywort Hydrocotyle ranunculoides Apiaceae native caraway-leaved lomatium Lomatium caruifolium var. caruifolium Apiaceae native woolly-fruited lomatium Lomatium dasycarpum dasycarpum Apiaceae native large-fruited lomatium Lomatium macrocarpum Apiaceae native common lomatium Lomatium utriculatum Apiaceae native Pacific oenanthe Oenanthe sarmentosa Apiaceae native 1 Stanford Working Plant List 1/15/08 wood sweet cicely Osmorhiza berteroi Apiaceae native mountain sweet cicely Osmorhiza chilensis Apiaceae native Gairdner's yampah (List 4) Perideridia gairdneri gairdneri Apiaceae -
Molecular Genetic Studies in Flax (Linum Usitatissimum L.)
Molecular genetic studies in flax (Linum usitatissimum L.) Moleculair genetische studies in vlas (Linum usitatissimum L.) Promotor: Prof. dr. ir. P. Stam Hoogleraar in de Plantenveredeling Copromotor: Dr. ir. H.J. van Eck Universitair docent, Laboratorium voor plantenveredeling Promotiecommissie: Prof. dr. A.G.M. Gerats (Radboud Universiteit Nijmegen) Prof. dr. R.F. Hoekstra (Wageningen Universiteit) Dr. R.G. van den Berg (Wageningen Universiteit) Dr. ir. J.W. van Ooijen (Kyazma B.V., Wageningen) Dit onderzoek is uitgevoerd binnen de onderzoeksscholen ‘Experimental Plant Sciences’ en ‘Production Ecology and Resource Conservation’. Molecular genetic studies in flax (Linum usitatissimum L.) Jaap Vromans Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, Prof. Dr. M.J. Kropff, in het openbaar te verdedigen op woensdag 15 maart 2006 des namiddags te vier uur in de Aula CIP-DTA Koninklijke Bibliotheek, Den Haag Molecular genetic studies in flax (Linum usitatissimum L.) Jaap Vromans PhD thesis, Wageningen University, The Netherlands With references – with summaries in Dutch and English ISBN 90-8504-374-3 Contents Chapter 1 General introduction 7 Chapter 2 Molecular analysis of flax (Linum usitatissimum L.) reveals a narrow genetic basis of fiber flax 21 Chapter 3 The molecular genetic variation in the genus Linum 41 Chapter 4 Selection of highly polymorphic AFLP fingerprints in flax (Linum usitatissimum L.) reduces the workload in genetic linkage map construction and results in -

Evolutionary History of Floral Key Innovations in Angiosperms Elisabeth Reyes
Evolutionary history of floral key innovations in angiosperms Elisabeth Reyes To cite this version: Elisabeth Reyes. Evolutionary history of floral key innovations in angiosperms. Botanics. Université Paris Saclay (COmUE), 2016. English. NNT : 2016SACLS489. tel-01443353 HAL Id: tel-01443353 https://tel.archives-ouvertes.fr/tel-01443353 Submitted on 23 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NNT : 2016SACLS489 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY, préparée à l’Université Paris-Sud ÉCOLE DOCTORALE N° 567 Sciences du Végétal : du Gène à l’Ecosystème Spécialité de Doctorat : Biologie Par Mme Elisabeth Reyes Evolutionary history of floral key innovations in angiosperms Thèse présentée et soutenue à Orsay, le 13 décembre 2016 : Composition du Jury : M. Ronse de Craene, Louis Directeur de recherche aux Jardins Rapporteur Botaniques Royaux d’Édimbourg M. Forest, Félix Directeur de recherche aux Jardins Rapporteur Botaniques Royaux de Kew Mme. Damerval, Catherine Directrice de recherche au Moulon Président du jury M. Lowry, Porter Curateur en chef aux Jardins Examinateur Botaniques du Missouri M. Haevermans, Thomas Maître de conférences au MNHN Examinateur Mme. Nadot, Sophie Professeur à l’Université Paris-Sud Directeur de thèse M. -

Vegetative Growth and Organogenesis 555
Vegetative Growth 19 and Organogenesis lthough embryogenesis and seedling establishment play criti- A cal roles in establishing the basic polarity and growth axes of the plant, many other aspects of plant form reflect developmental processes that occur after seedling establishment. For most plants, shoot architecture depends critically on the regulated production of determinate lateral organs, such as leaves, as well as the regulated formation and outgrowth of indeterminate branch systems. Root systems, though typically hidden from view, have comparable levels of complexity that result from the regulated formation and out- growth of indeterminate lateral roots (see Chapter 18). In addition, secondary growth is the defining feature of the vegetative growth of woody perennials, providing the structural support that enables trees to attain great heights. In this chapter we will consider the molecular mechanisms that underpin these growth patterns. Like embryogenesis, vegetative organogenesis and secondary growth rely on local differences in the interactions and regulatory feedback among hormones, which trigger complex programs of gene expres- sion that drive specific aspects of organ development. Leaf Development Morphologically, the leaf is the most variable of all the plant organs. The collective term for any type of leaf on a plant, including struc- tures that evolved from leaves, is phyllome. Phyllomes include the photosynthetic foliage leaves (what we usually mean by “leaves”), protective bud scales, bracts (leaves associated with inflorescences, or flowers), and floral organs. In angiosperms, the main part of the foliage leaf is expanded into a flattened structure, the blade, or lamina. The appearance of a flat lamina in seed plants in the middle to late Devonian was a key event in leaf evolution. -

Honey Bee Suite © Rusty Burlew 2015 Master Plant List by Scientific Name United States
Honey Bee Suite Master Plant List by Scientific Name United States © Rusty Burlew 2015 Scientific name Common Name Type of plant Zone Full Link for more information Abelia grandiflora Glossy abelia Shrub 6-9 http://plants.ces.ncsu.edu/plants/all/abelia-x-grandiflora/ Acacia Acacia Thorntree Tree 3-8 http://www.2020site.org/trees/acacia.html Acer circinatum Vine maple Tree 7-8 http://www.nwplants.com/business/catalog/ace_cir.html Acer macrophyllum Bigleaf maple Tree 5-9 http://treesandshrubs.about.com/od/commontrees/p/Big-Leaf-Maple-Acer-macrophyllum.htm Acer negundo L. Box elder Tree 2-10 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=a841 Acer rubrum Red maple Tree 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=275374&isprofile=1&basic=Acer%20rubrum Acer rubrum Swamp maple Tree 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=275374&isprofile=1&basic=Acer%20rubrum Acer saccharinum Silver maple Tree 3-9 http://en.wikipedia.org/wiki/Acer_saccharinum Acer spp. Maple Tree 3-8 http://en.wikipedia.org/wiki/Maple Achillea millefolium Yarrow Perennial 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=b282 Aesclepias tuberosa Butterfly weed Perennial 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=b490 Aesculus glabra Buckeye Tree 3-7 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=281045&isprofile=1&basic=buckeye