Fall 2006 Gems & Gemology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Gemstones and Geosciences in Space and Time Digital Maps to the “Chessboard Classification Scheme of Mineral Deposits”
Earth-Science Reviews 127 (2013) 262–299 Contents lists available at ScienceDirect Earth-Science Reviews journal homepage: www.elsevier.com/locate/earscirev Gemstones and geosciences in space and time Digital maps to the “Chessboard classification scheme of mineral deposits” Harald G. Dill a,b,⁎,BertholdWeberc,1 a Federal Institute for Geosciences and Natural Resources, P.O. Box 510163, D-30631 Hannover, Germany b Institute of Geosciences — Gem-Materials Research and Economic Geology, Johannes-Gutenberg-University, Becherweg 21, D-55099 Mainz, Germany c Bürgermeister-Knorr Str. 8, D-92637 Weiden i.d.OPf., Germany article info abstract Article history: The gemstones, covering the spectrum from jeweler's to showcase quality, have been presented in a tripartite Received 27 April 2012 subdivision, by country, geology and geomorphology realized in 99 digital maps with more than 2600 mineral- Accepted 16 July 2013 ized sites. The various maps were designed based on the “Chessboard classification scheme of mineral deposits” Available online 25 July 2013 proposed by Dill (2010a, 2010b) to reveal the interrelations between gemstone deposits and mineral deposits of other commodities and direct our thoughts to potential new target areas for exploration. A number of 33 categories Keywords: were used for these digital maps: chromium, nickel, titanium, iron, manganese, copper, tin–tungsten, beryllium, Gemstones fl Country lithium, zinc, calcium, boron, uorine, strontium, phosphorus, zirconium, silica, feldspar, feldspathoids, zeolite, Geology amphibole (tiger's eye), olivine, pyroxenoid, garnet, epidote, sillimanite–andalusite, corundum–spinel−diaspore, Geomorphology diamond, vermiculite–pagodite, prehnite, sepiolite, jet, and amber. Besides the political base map (gems Digital maps by country) the mineral deposit is drawn on a geological map, illustrating the main lithologies, stratigraphic Chessboard classification scheme units and tectonic structure to unravel the evolution of primary gemstone deposits in time and space. -

STRONG and WEAK INTERLAYER INTERACTIONS of TWO-DIMENSIONAL MATERIALS and THEIR ASSEMBLIES Tyler William Farnsworth a Dissertati
STRONG AND WEAK INTERLAYER INTERACTIONS OF TWO-DIMENSIONAL MATERIALS AND THEIR ASSEMBLIES Tyler William Farnsworth A dissertation submitted to the faculty at the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Chemistry. Chapel Hill 2018 Approved by: Scott C. Warren James F. Cahoon Wei You Joanna M. Atkin Matthew K. Brennaman © 2018 Tyler William Farnsworth ALL RIGHTS RESERVED ii ABSTRACT Tyler William Farnsworth: Strong and weak interlayer interactions of two-dimensional materials and their assemblies (Under the direction of Scott C. Warren) The ability to control the properties of a macroscopic material through systematic modification of its component parts is a central theme in materials science. This concept is exemplified by the assembly of quantum dots into 3D solids, but the application of similar design principles to other quantum-confined systems, namely 2D materials, remains largely unexplored. Here I demonstrate that solution-processed 2D semiconductors retain their quantum-confined properties even when assembled into electrically conductive, thick films. Structural investigations show how this behavior is caused by turbostratic disorder and interlayer adsorbates, which weaken interlayer interactions and allow access to a quantum- confined but electronically coupled state. I generalize these findings to use a variety of 2D building blocks to create electrically conductive 3D solids with virtually any band gap. I next introduce a strategy for discovering new 2D materials. Previous efforts to identify novel 2D materials were limited to van der Waals layered materials, but I demonstrate that layered crystals with strong interlayer interactions can be exfoliated into few-layer or monolayer materials. -

New Occurrences from the Postmasburg Manganese Field, Northern Cape Province, South Africa
981 The Canadian Mineralogist Vol. 53, pp. 981-990 (2015) DOI: 10.3749/canmin.1500063 TOKYOITE, As-RICH TOKYOITE, AND NOÉLBENSONITE: NEW OCCURRENCES FROM THE POSTMASBURG MANGANESE FIELD, NORTHERN CAPE PROVINCE, SOUTH AFRICA GELU COSTIN Department of Geology, Rhodes University, P.O. Box 94, Grahamstown 6140, South Africa Department of Earth Science, MS-126 Rice University, 6100 Main Street, Houston, Texas 77005, USA § BRENTON FAIREY AND HARILAOS TSIKOS Department of Geology, Rhodes University, P.O. Box 94, Grahamstown 6140, South Africa ARNOLD GUCSIK Department of Geology, University of Johannesburg, Johannesburg, South Africa ABSTRACT Samples of manganese-rich rock containing two compositional varieties of tokyoite in association with noélbensonite were retrieved from a drill core obtained from the Postmasburg area in the Northern Cape Province of South Africa. The samples consist of fine-grained braunite, hematite, and hausmannite. Within this material abundant vugs are observed that are filled with witherite, baryte, and barytocalcite. In addition, As-rich tokyoite, tokyoite, and noélbensonite occur in the center of the vugs, in fine aggregates 0.1 to 1 mm in size. Individual As-rich tokyoite grains are typically 20–200 mm in size. The outer walls of the vugs are lined with microcrystalline K-feldspar, witherite, and/or sérandite. Textural evidence of the Ba-rich mineral phases in association with As-rich tokyoite suggest an epigenetic mode of formation of the observed assemblages, caused by V- and As-bearing alkali-rich fluids interacting with pre-existing Mn-rich minerals. Electron microprobe analysis (EMPA), electron backscatter diffraction analysis (EBSD), and Raman spectroscopy show that the As-rich tokyoite is a mineral belonging to the brackebuschite mineral group. -

Download the Scanned
INDEX TO VOLUME 24 Leading articles are in bold face type; notes, abstracts and reviews are in ordinary type. Only minerals for which definite data are siven are indexed. Abukumalite(ShinHata)....... 66 Brandenberger, E. Angewandte Adinolesof D.inasHead, Cornwall. Kristallstrukturlehre. IBook (Agrell). 63 review] 276 Agrell,S. O.. 63 Bray, J. M. Ilmenite-hematite- Alderman, A 277 magnetite relations in some Allen,V. T.. 194 emery ores. 162,183 Alurgite from California, new oc- Brochantite. (Palache,Richmond) 463 currenceof. (Webb). I z.t Brunckite. (Herzenberg) 350 Ammonium mica synthesized from Buerger,M. J. Crystal structure of vermiculite. (Gruner) 428 gudmundite.. 183 Anderson,B. W... 528 Bullard, F. M. Rosebudmeteorite, AngewandteKristallstrukturleh re. Milam Co., Texas 184,242 (Brandenberger)[Book review] 276 Burns,B. D. 528 Antlerite. (Palache). 293 Apatite, fluorescent,from Center Calcite,Fe-Mn. (Yosimura). 660 Strafford, N. Hamp. (Stewart) 274 Caledonite. (Palache, Richmond) 441 Australitesof unusualform. (Single- Canyon Diablo iron, identification ton). 63 of diamond in. (Ksanda, Awariute.(Owens, Burns). 528 Henderson).... 677 CesAro,G..... 280 "Baddelyite" from Alno-an error. Chao,S.H...... 277 (vonEckermann). 528 Chemicalanalyses of minerals,pres- Bannister,F. A. ....64,66 entationof. (Hey). 347 Barksdale, J. D. Silicified wood in Chlorite veins in serpentine near dolomite. .. ..181,699 Kings River, Calif. (Durrell, Bavenite,ne\\r occurrence of. (Clar- Macdonald). 452 ingbull). 277 Chrysoberyl occurrence near Behre,C. H., Jr. 181 Golden, Colo. (Waldschmidt, Beliankin,D. S.. 279 Gaines). 193,267 Bell,J. F. 181 Cinnabar,darkening of, in sunlight. Berman, H. Torsion microbalance (Dreyer).. +57 for determining specific grav- Claringbull,G. F.. 277,347 ity of minerals 182,434 Clay, Hawaiian ceramic, mineral -- and Gonyer, F, A. -

Green Aventurine Quartz: Mineralogical Characterization
Volume 20 No. 2 April 1986 GAGTL EDU Set 3 ^eJournal of Gemmology GEMMOLOGICAL ASSOCIATION OF GREAT BRITAIN OFFICERS AND COUNCIL President: Sir Frank Claringbull Ph.D., F.InsLP., F.G.S. Vice-Presidents: J. R. H. Chisholm, M.A., F.G .A. R. K. Mitchell, F.G.A. H. J. Wheeler, F.G.A. Chairman: D. J. Callaghan, F. G. A. Vice-Chairman: N. W. Deeks, F.G.A. Honorary Treasurer: N. B. Israel, F.G.A. Fellows elected to Council: C. R. Cavey B. Jackson M. J. O'Donoghue, L. F. Cole C. B. Jones M. A., F.G.S. R. W. Croydon D. M. Larcher P. G. Read, C.Eng., P. J. E. Daly, B.Sc. D. Morgan M.I.E.E., M.LE.R.E. A. J. French G. Neary A. W. R. Round S. E. Hiscox J. B. Nelson, Ph.D., F.R.M.S., E. Stern J. A. W. Hodgkinson F. InsLP., F.G.S. C. H. Winter D. Inkersole W. Nowak, C.Eng., F.R.Ac.S. Branch Chairmen: Midlands Branch: C. L. Hundy, F.G.A. North-West Branch: S. Hill, F.G.A. Sowh Yorkshire & Distria Branch: J. I. Reynolds, F.G.A. Examiners: E. A. Jobbins,B.Sc., F.I.M.M., F.G.A. A. J. Allnutt, M.Se.,Ph.D., F.G.A. R. R. Harding, B.Sc., D.Phil., F.G.A. G. H. Jones, B.Se., Ph.D., F.G.A. D. G. Kent, F.G.A. E. M. Bruton, F.G.A. P. Sadler, B.Sc., F.G.S., F.G.A. -

UCLA Electronic Theses and Dissertations
UCLA UCLA Electronic Theses and Dissertations Title Mineralogic controls on the infrared stimulated luminescence of feldspars: an exploratory study of the effects of Al,Si order and composition on the behavior of a modified post-IR IRSL signal Permalink https://escholarship.org/uc/item/9m86t9pq Author Daniels, JILLIAN Publication Date 2016 Supplemental Material https://escholarship.org/uc/item/9m86t9pq#supplemental Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles Mineralogic controls on the infrared stimulated luminescence of feldspars: an exploratory study of the effects of Al,Si order and composition on the behavior of a modified post-IR IRSL signal A thesis submitted in partial satisfaction of the requirements for the degree Master of Science in Geology by JILLIAN THERESA Daniels 2016 ABSTRACT OF THE THESIS Mineralogic controls on the infrared stimulated luminescence of feldspars: an exploratory study of the effects of Al,Si order and composition on the behavior of a modified post-IR IRSL signal by JILLIAN THERESA Daniels Master of Science in Geology University of California, Los Angeles, 2016 Professor Kevin D McKeegan, Chair The luminescence characteristics of a variety of feldspars were investigated using the post-IR IRSL signal to facilitate the understanding of the origins and behavior of signals used in luminescence dating. These feldspars were characterized in terms of major composition, Al,Si ordering, and minor impurities, and compared to the luminescence decay parameters of brightness, bleachability, and kinetic order calculated from the pIRIR decay signal. The behavior of these parameters over varying preheat temperatures, pIRIR measurement temperatures, IR50 measurement times, and first IR measurement temperatures were analyzed. -

New Mineral Names*
American Mineralogist, Volume 90, pages 1466–1469, 2005 New Mineral Names* PAULA C. PIILONEN† AND T. SCOTT ERCIT‡ Research Division, Canadian Museum of Nature, P.O. Box 3443, Stn. D, Ottawa, Ontario K1P 6P4, Canada CENTROSYMMETRIC ANALOGUE OF LABYRINTHITE NALDRETTITE* K.A. Rosenberg, R.K. Rastsvetayeva, N.V. Chukanov, I.A. Verin L.J. Cabri, A.M. McDonald, C.J. Stanley, N.S. Rudashevsky, (2004) Centrosymmetric modular structure of an analogue of G. Poirier, B.R. Durham, J.E. Mungall, V.N. Rudashevsky labyrinthite. Dokl. Akad. Nauk, 399, 791–794 (in Russian); (2005) Naldrettite, Pd2Sb, a new intermetallic mineral from Dokl. Chem., 399, 253–256 (in English). the Mesamax Northwest deposit, Ungava region, Québec, Canada. Mineral. Mag., 69, 89–97. An apparently new member of the eudialyte group has been found in the central zone of an alkaline pegmatite at Mt. Naldrettite occurs as anhedral grains from 10 to 239 µm Koashva, Khibiny massif, Kola Peninsula, Russia. The mineral (average 74.4 µm). Grains were liberated and isolated from the occurs in tabular grains intimately intergrown with villiaumite, in matrix by hydroseparation. It is often found attached to sulÞ de associations with lomonosovite, barytolamprophyllite, aegirine, minerals and commonly associated with clinochlore. The min- and microcline. The authors infer from this assemblage that the eral is metallic and opaque. It does not show cleavage, has an formational conditions included relatively high temperature and irregular fracture, a ductile behavior (ß exibly inelastic), and has high sodium and ß uorine activity. The mineral is dichroic brown- a Mohs hardness of 4 to 5 (average VHN50 load of 393 in the gray to raspberry-pink. -

Genesis of Karst-Hosted Manganese Ores of the Postmasburg Manganese Field, South Africa with Emphasis on Evidence for Hydrothermal Processes
Genesis of karst-hosted manganese ores of the Postmasburg Manganese Field, South Africa with emphasis on evidence for hydrothermal processes A thesis submitted in fulfilment of the requirements for the degree of MASTER OF SCIENCE of RHODES UNIVERSITY by Brenton John Fairey June 2013 Abstract The Postmasburg Manganese Field (PMF), located in the Northern Cape Province of South Africa, once represented one of the largest sources of manganese ore worldwide. However, the discovery of the giant manganese deposits of the Kalahari Manganese Field (KMF) led to the gradual decline in manganese mining activity in the PMF. Two belts of manganese ore deposits have been distinguished in the PMF, namely the Western Belt of ferruginous manganese ores and the Eastern Belt of siliceous manganese ores. Prevailing models of ore formation in these two belts invoke karstification of manganese-rich dolomites and residual accumulation of manganese wad which later underwent diagenetic and low-grade metamorphic processes. For the most part, the role of hydrothermal processes in ore formation and metasomatic alteration is not addressed. The identification of an abundance of common and some rare Al-, Na-, K- and Ba-bearing minerals, particularly aegirine, albite, microcline, banalsite, sérandite-pectolite, paragonite and natrolite in the PMF ores studied in this thesis, is indicative of the influence of hydrothermal activity. Enrichments in Na, K and/or Ba in the ores are generally on a percentage level for the majority of samples analysed through bulk-rock techniques. The discovery of a Ba-Mn arsenate/vanadate similar to gamagarite may also indicate that the hydrothermal fluid affecting the ores was not only alkali-rich but also probably contained some As and V. -

The Crystallographic Investigation of a Strontium Labradorite
The Crystallographic investigation ot a Strontium Labradorite B.Sc. Thesis by George CORDAHI ABSTRACT Precession photography was used to determine the lattice parame ters. the crystal system, the spaoe group and the structure of an art1f1c1al Sr-labradorite of compos1tion:Ab27, SrAn?J. The lattice parameters determined are : a•8.361~. b= 1J.020A, c= 7. 1 orA·. Q( = ~ = 90• • ~ = 115.8J4D • The crystal system is mon oc11n1c, space group = C2/m and structure is albite type, reflec tions being restricted to the 1 a 1 type. The abundance, lithoph 1le characteristics and appropriate ionic radii of elements in Groups IAand IIA are the factors governing their presence as cations of feldspars in nature. The structure of feldspars are discussed as a function of the relative proportion of cations of a charge of +l and +2. The crystal symmetry (i.e. monoclinicity or triclinicity) is discussed as a function of the ionic radius of the cation. 1 Introduction: The purpose or the present work was to attempt to correlate the structure, crystal chemistry and lattice parameters ot a Sr-Plag 1oclase of known composit~on with other members or the feldspar group or minerals. Particular attention is given to: 1) Determination of the space group and lattice parameters or the Sr-feldspar crystal. 2) Prediction of the crystal structure or a hypothetical pure Sr-feldspar, and )) to comment on the extent of ionic substitution of Ca, Na,K and Ba in 'sr feldspars. (i.e. the amount or solid solution between these end members) Ce.~sio..'l\ ll~ato\'kn.e KM$i.0 0s A subsequent refinement of the structure of the Sr-plag1oclase by H.D. -

Mineralogy and Museums
SIXTH INTERNATIONAL CONFERENCE M&M6MINERALOGY AND MUSEUMS Golden, Colorado U.S.A. PROGRAM September 7–9, 2008 & ABSTRACTS 2008 — SIXTH INTERNATIONAL CONFERENCE MINERALOGY AND MUSEUMS COLORADO SCHOOL OF MINES • GOLDEN, CO PROGRAM & ABSTRACTS SEPTEMBER 7-9, 2008 M&M6 2008 — SIXTH INTERNATIONAL CONFERENCE COLORADO SCHOOL OF MINES • GOLDEN COLORADO USA • SEPTEMBER 7-9, 2008 MINERALOGY AND MUSEUMS TABLE OF CONTENTS The Organizing General Information ................................................................................................................................................................................ 4 Committee gratefully Scientific Information ............................................................................................................................................................................. 5 acknowledges Technical Session Program ................................................................................................................................................................... 6 the generous support Index by Author ...................................................................................................................................................................................... 10 Oral Presentation Abstracts ................................................................................................................................................................ 11 of our sponsors: Poster Presentation Abstracts ........................................................................................................................................................... -
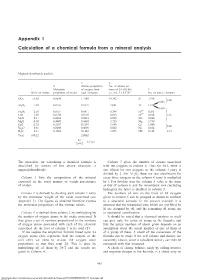
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

The Microscopic Determination of the Nonopaque Minerals
DEPARTMENT OF THE INTERIOR ALBERT B. FALL, Secretary UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, Director Bulletin 679 THE MICROSCOPIC DETERMINATION OF THE NONOPAQUE MINERALS BY ESPER S. LARSEN WASHINGTON GOVERNMENT PRINTING OFFICE 1921 CONTENTS. CHAPTER I. Introduction.................................................. 5 The immersion method of identifying minerals........................... 5 New data............................................................. 5 Need of further data.................................................... 6 Advantages of the immersion method.................................... 6 Other suggested uses for the method.................................... 7 Work and acknowledgments............................................. 7 CHAPTER II. Methods of determining the optical constants of minerals ....... 9 The chief optical constants and their interrelations....................... 9 Measurement of indices of refraction.................................... 12 The embedding method............................................ 12 The method of oblique illumination............................. 13 The method of central illumination.............................. 14 Immersion media.................................................. 14 General features............................................... 14 Piperine and iodides............................................ 16 Sulphur-selenium melts....................................... 38 Selenium and arsenic selenide melts........................... 20 Methods of standardizing