Cerebellar Climbing Fibers Modulate Simple Spikes in Purkinje Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets
THE JOURNAL OF COMPARATIVE NEUROLOGY 369~345-360 ( 1996) Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets DIANA L. WEEDMAN, TAN PONGSTAPORN, AND DAVID K. RYUGO Center for Hearing Sciences, Departments of Otolaryngoloby-Head and Neck Surgery and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, Maryland 2 1205 ABSTRACT The principal projection neurons of the cochlear nucleus receive the bulk of their input from the auditory nerve. These projection neurons reside in the core of the nucleus and are surrounded by an external shell, which is called the granule cell domain. Interneurons of the cochlear granule cell domain are the target for nonprimary auditory inputs, including projections from the superior olivary complex, inferior colliculus, and auditory cortex. The granule cell domain also receives projections from the cuneate and trigeminal nuclei, which are first-order nuclei of the somatosensory system. The cellular targets of the nonprimary projections are mostly unknown due to a lack of information regarding postsynaptic profiles in the granule cell areas. In the present paper, we examined the synaptic relationships between a heterogeneous class of large synaptic terminals called mossy fibers and their targets within subdivisions of the granule cell domain known as the lamina and superficial layer. By using light and electron microscopic methods in these subdivisions, we provide evidence for three different neuron classes that receive input from the mossy fibers: granule cells, unipolar brush cells, and a previously undescribed class called chestnut cells. The distinct synaptic relations between mossy fibers and members of each neuron class further imply fundamentally separate roles for processing acoustic signals. -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -

Three-Dimensional Comparison of Ultrastructural Characteristics at Depressing and Facilitating Synapses Onto Cerebellar Purkinje Cells
The Journal of Neuroscience, September 1, 2001, 21(17):6666–6672 Three-Dimensional Comparison of Ultrastructural Characteristics at Depressing and Facilitating Synapses onto Cerebellar Purkinje Cells Matthew A. Xu-Friedman,1 Kristen M. Harris,2 and Wade G. Regehr1 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115, and 2Department of Biology, Boston University, Boston, Massachusetts 02215 Cerebellar Purkinje cells receive two distinctive types of exci- vesicles per release site to test whether this number determines tatory inputs. Climbing fiber (CF) synapses have a high proba- the probability of release and synaptic plasticity. PF and CF bility of release and show paired-pulse depression (PPD), synapses had the same number of anatomically docked vesi- whereas parallel fiber (PF) synapses facilitate and have a low cles (7–8). The number of docked vesicles at the CF does not probability of release. We examined both types of synapses support a simple model of PPD in which release of a single using serial electron microscopic reconstructions in 15-d-old vesicle during the first pulse depletes the anatomically docked rats to look for anatomical correlates of these differences. PF vesicle pool at a synapse. Alternatively, only a fraction of ana- and CF synapses were distinguishable by their overall ultra- tomically docked vesicles may be release ready, or PPD could structural organization. There were differences between PF and result from multivesicular release at each site. Similarities in the CF synapses in how many release sites were within 1 mofa number of docked vesicles for PF and CF synapses indicate mitochondrion (67 vs 84%) and in the degree of astrocytic that differences in probability of release are unrelated to the ensheathment (67 vs 94%). -

Cerebellar Cortical Neuron Responses Evoked from the Spinal Border Cell Tract
Cerebellar cortical neuron responses evoked from the spinal border cell tract. Geborek, Pontus; Spanne, Anton; Bengtsson, Fredrik; Jörntell, Henrik Published in: Frontiers in Neural Circuits DOI: 10.3389/fncir.2013.00157 2013 Link to publication Citation for published version (APA): Geborek, P., Spanne, A., Bengtsson, F., & Jörntell, H. (2013). Cerebellar cortical neuron responses evoked from the spinal border cell tract. Frontiers in Neural Circuits, 7, [157]. https://doi.org/10.3389/fncir.2013.00157 Total number of authors: 4 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 Download date: 04. Oct. 2021 ORIGINAL RESEARCH ARTICLE published: 08 October 2013 NEURAL CIRCUITS doi: 10.3389/fncir.2013.00157 Cerebellar cortical neuron responses evoked from the spinal border cell tract Pontus Geborek, Anton Spanne, Fredrik Bengtsson and Henrik Jörntell* Neural Basis of Sensorimotor Control, Department of Experimental Medical Science, Lund University, Lund, Sweden Edited by: Spinocerebellar systems are likely to be crucial for cerebellar hallmark functions such Egidio D’Angelo, University of Pavia, as coordination. -

Cerebellar Histology & Circuitry
Cerebellar Histology & Circuitry Histology > Neurological System > Neurological System CEREBELLAR HISTOLOGY & CIRCUITRY SUMMARY OVERVIEW Gross Anatomy • The folding of the cerebellum into lobes, lobules, and folia allows it to assume a tightly packed, inconspicuous appearance in the posterior fossa. • The cerebellum has a vast surface area, however, and when stretched, it has a rostrocaudal expanse of roughly 120 centimeters, which allows it to hold an estimated one hundred billion granule cells — more cells than exist within the entire cerebral cortex. - It is presumed that the cerebellum's extraordinary cell count plays an important role in the remarkable rehabilitation commonly observed in cerebellar stroke. Histology Two main classes of cerebellar nuclei • Cerebellar cortical neurons • Deep cerebellar nuclei CEREBELLAR CORTICAL CELL LAYERS Internal to external: Subcortical white matter Granule layer (highly cellular) • Contains granule cells, Golgi cells, and unipolar brush cells. Purkinje layer 1 / 9 • Single layer of large Purkinje cell bodies. • Purkinje cells project a fine axon through the granule cell layer. - Purkinje cells possess a large dendritic system that arborizes (branches) extensively and a single fine axon. Molecular layer • Primarily comprises cell processes but also contains stellate and basket cells. DEEP CEREBELLAR NUCLEI From medial to lateral: Fastigial Globose Emboliform Dentate The globose and emboliform nuclei are also known as the interposed nuclei • A classic acronym for the lateral to medial organization of the deep nuclei is "Don't Eat Greasy Food," for dentate, emboliform, globose, and fastigial. NEURONS/FUNCTIONAL MODULES • Fastigial nucleus plays a role in the vestibulo- and spinocerebellum. • Interposed nuclei are part of the spinocerebellum. • Dentate nucleus is part of the pontocerebellum. -

2141.Full.Pdf
The Journal of Neuroscience, June 1989, g(8): 2141-2150 Physiological and Anatomical Studies of the Interactions Between Purkinje Cells and Basket Cells in the Cat’s Cerebellar Cortex: Evidence for a Unitary Relationship Daniel L. O’Donoghue,a James S. King, and Georgia A. Bishop Department of Anatomy and Neuroscience Program, The Ohio State University, Columbus, Ohio 43210 Intracellular recordings have been obtained from neurons in tions of these units with HRP revealed that the responseswere lobule V of the cat’s vermis, which were identified as basket obtained from basket cells. cells following intracellular injections of HRP. Stimulation of Basket cells are interneurons that are located in the lower the inferior cerebellar peduncle or peripheral nerves elicited molecular layer of the cerebellar cortex (Fox et al., 1967; Mug- an initial depolarizing and subsequent hyperpolarizing re- naini, 1972; Palay and Chan-Palay, 1974). Their somata are sponse. Neither potential could be graded with changes in interspersedbetween and within the dendritic trees of Purkinje stimulus intensity; both displayed all-or-none properties at cells (Fox et al., 1967; Palay and Chan-Palay, 1974); their den- threshold levels of stimulation. The depolarization and hy- drites extend toward the pial surface, and their axons are pri- perpolarization were confirmed as an excitatory postsyn- marily oriented in the sagittal plane. Descendingcollaterals of aptic potential and an inhibitory postsynaptic potential (IPSP), theseaxons form a unique pericellular basket around the somata respectively, on the basis of their response to intracellular and axon hillocks of Purkinje cells, defined as the pinceau by injections of hyperpolarizing and depolarizing currents into Ram6n y Cajal (19 11). -

Synaptic Remodeling Between Climbing Fibers and Purkinje Cells in the Developing Cerebellum Begins with Positive Feedback Addition of Synapses
bioRxiv preprint doi: https://doi.org/10.1101/627299; this version posted May 3, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. The rich get richer: synaptic remodeling between climbing fibers and Purkinje cells in the developing cerebellum begins with positive feedback addition of synapses. AUTHORS Alyssa Michelle Wilson1*✝, Richard Schalek2, Adi Suissa-Peleg2,3, Thouis Ray Jones4, Seymour Knowles-Barley5, Hanspeter Pfister3, Jeff William Lichtman6*. 1Department of Physics, Harvard University, Cambridge, MA 02138, USA 2Center for Brain Science, Harvard University, Cambridge, MA 02138, USA 3School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA 4Broad Institute, Cambridge, MA 02142, USA 5Volpara Health Technologies, Wellington, 6011, New Zealand 6Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138, USA *Correspondence: [email protected] (A.M.W.), [email protected] (J.W.L.) ✝Lead Contact SUMMARY During postnatal development, cerebellar climbing fibers strongly innervate a subset of their original Purkinje cell targets and eliminate their connections from the rest. In the adult, each climbing fiber innervates a small number of Purkinje cells and each Purkinje cell is innervated by a single climbing fiber. To get insight about the processes responsible for this remapping, we reconstructed serial electron microscopy datasets from mice during the first postnatal week. In contrast to adult connectivity, individual neonatal climbing fibers innervate many nearby Purkinje cells, and multiple climbing fibers innervate each Purkinje cell. -

Multiple Climbing Fibers Signal to Molecular Layer Interneurons
ARTICLES Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover Germa´n Szapiro & Boris Barbour Spillover of glutamate under physiological conditions has only been established as an adjunct to conventional synaptic transmission. Here we describe a pure spillover connection between the climbing fiber and molecular layer interneurons in the rat cerebellar cortex. We show that, instead of acting via conventional synapses, multiple climbing fibers activate AMPA- and NMDA- type glutamate receptors on interneurons exclusively via spillover. Spillover from the climbing fiber represents a form of glutamatergic volume transmission that could be triggered in a regionalized manner by experimentally observed synchronous climbing fiber activity. Climbing fibers are known to direct parallel fiber synaptic plasticity in interneurons, so one function of this spillover is likely to involve controlling synaptic plasticity. http://www.nature.com/natureneuroscience Spillover of neurotransmitter—action at synaptic contacts other than unknown type of axodendritic (or axosomatic) contact17. Despite those where it was released—has proved to be a controversial subject. extensive and tight apposition between axonal (climbing fiber) and For simple, small glutamatergic synapses, spillover has only rarely been somatodendritic (interneuron) membranes, the apparent absence of reported1,2 at activity levels that are physiologically plausible, and vesicles and glutamate receptors argues against classical synaptic modeling suggests that the spillover of glutamate at simple synapses transmission occurring at these junctions. is probably not significant3 (but see ref. 4). It has been argued that Here we confirm the existence of the climbing fiber–interneuron spillover would reduce synaptic independence and thus reduce the connection and characterize its mechanism of transmission. -
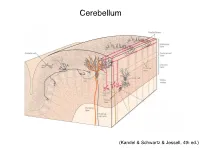
Cerebellum and Activation of the Cerebellum IO ST During Nonmotor Tasks
Cerebellum (Kandel & Schwartz & Jessell, 4th ed.) Granule186 cells are irreducibly smallChapter (6-8 7 μm) stellate cell basket cell outer synaptic layer PC rs cap PC layer grc Go inner 6 μm synaptic layer Figure 7.15 Largest cerebellar neuron occupies more than a 1,000-fold greater volume than smallest neuron. Thin section (~1 μ m) through monkey cerebellar cortex. Purkinje cell body (PC) and nucleus are far larger than those of granule cell (grc). The latter cluster to leave space for mossy fiber terminals to form glomeruli with grc dendritic claws and space for Golgi cells (Go). Note rich network of capillaries (cap). Fine, scat- tered dots are mitochondria. Courtesy of E. Mugnaini. brain ’ s most numerous neuron, this small cost grows large (see also chapter 13). Much of the inner synaptic layer is occupied by the large axon terminals of mossy fibers (figures 7.1 and 7.16). A terminal interlaces with multiple (~15) dendritic claws, each from a different but neighboring granule cell, and forms a complex knot (glomerulus ), nearly as large as a granule cell body (figures 7.1 and 7.16). The mossy fiber axon fires at an unusually high mean rate (up to 200 Hz) and is therefore among the brain’ s thickest (figure 4.6). To match the axon’ s high rate, a terminal expresses 150 active zones, 10 per postsynaptic granule cell (figure 7.16). These sites are capable of driving … and most expensive. 190 Chapter 7 10 4 8 3 9 19 10 10 × 6 × 2 2 4 ATP/s/cell ATP/s/cell ATP/s/m 1 2 0 0 astrocyte astrocyte Golgi cell Golgi cell basket cell basket cell stellate cell stellate cell granule cell granule cell mossy fiber mossy fiber Purkinje cell Purkinje Purkinje cell Purkinje Bergman glia Bergman glia climbing fiber climbing fiber Figure 7.18 Energy costs by cell type. -

The Modifiable Neuronal Network of the Cerebellum
Japanese Journal of Physiology, 34, 781-792, 1984 MINIREVIEW The Modifiable Neuronal Network of the Cerebellum M asao ITO Department of Physiology, Faculty of Medicine, University of Tokyo, Bunkyo-ku, Tokyo, 113 Japan An outstanding feature of the cerebellum known since the classic histological work of Ramon y Cajal is the relative simplicity and highly ordered geometry of its neuronal network. The cerebellar neuronal network consists of five major types of cortical neurons (Purkinje, basket, stellate, Golgi, and granule cells), two major types of afferents (mossy and climbing fibers), and the subcortical cells in the vestibular and cerebellar nuclei. When the neuronal network structure of the cerebellum was comprehensively dissected and described in great detail in the 1960s, and summarized by ECCLES,ITO, and SZ.ENTAGOTHAI[8], it was taken as a challenge to understand more completely the complex functions of the central nervous system using these fundamental studies of the brain as groundwork for future elucidation. As the gap between our understanding of the brain and our knowledge of neuronal networks is in general so large, hope has arisen that cere- bellar physiology may successfully fill the gap rather soon. Rigorous efforts using numerous newly-introduced techniques have been devoted during the past two decades, yielding remarkable progress that meets expectations at least to some extent. A number of problems remain to be solved, however, before we fully understand the cerebellum. It seems important at this point to review the major achievements of the past two decades in order to gain a clearer perspective of cerebellar physiology for the coming decade. -

The Long Journey of Pontine Nuclei Neurons : from Rhombic Lip To
REVIEW Erschienen in: Frontiers in Neural Circuits ; 11 (2017). - 33 published: 17 May 2017 http://dx.doi.org/10.3389/fncir.2017.00033 doi: 10.3389/fncir.2017.00033 The Long Journey of Pontine Nuclei Neurons: From Rhombic Lip to Cortico-Ponto-Cerebellar Circuitry Claudius F. Kratochwil 1,2, Upasana Maheshwari 3,4 and Filippo M. Rijli 3,4* 1Chair in Zoology and Evolutionary Biology, Department of Biology, University of Konstanz, Konstanz, Germany, 2Zukunftskolleg, University of Konstanz, Konstanz, Germany, 3Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland, 4University of Basel, Basel, Switzerland The pontine nuclei (PN) are the largest of the precerebellar nuclei, neuronal assemblies in the hindbrain providing principal input to the cerebellum. The PN are predominantly innervated by the cerebral cortex and project as mossy fibers to the cerebellar hemispheres. Here, we comprehensively review the development of the PN from specification to migration, nucleogenesis and circuit formation. PN neurons originate at the posterior rhombic lip and migrate tangentially crossing several rhombomere derived territories to reach their final position in ventral part of the pons. The developing PN provide a classical example of tangential neuronal migration and a study system for understanding its molecular underpinnings. We anticipate that understanding the mechanisms of PN migration and assembly will also permit a deeper understanding of the molecular and cellular basis of cortico-cerebellar circuit formation and function. Keywords: pontine gray nuclei, reticulotegmental nuclei, precerebellar system, cortico-ponto-cerebellar circuitry, Hox genes Edited by: Takao K. Hensch, INTRODUCTION Harvard University, United States Reviewed by: The basal pontine nuclei (BPN) (also known as basilar pons, pontine gray nuclei or pontine nuclei Masahiko Takada, (PN)) and the reticulotegmental nuclei (RTN) (also known as nucleus reticularis tegmenti pontis) Kyoto University, Japan are located within the ventral portion of the pons. -

The Organization of the Corticonuclear and Olivocerebellar Climbing Fiber
Journal of Neurocytology 33, 5–21 (2004) The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: The congruence of projection zones and the zebrin pattern JAN VOOGD∗ and TOMJ.H.RUIGROK Department of Neuroscience, Erasmus MC, Rotterdam, P.O. Box 1738, 3000 DR Rotterdam, The Netherlands [email protected] Received 23 May 2003; revised 2 September 2003; accepted 2 September 2003 Abstract The zonal organization of the corticonuclear and the olivocerebellar climbing fiber projections to the vermis of the cerebellum of the rat was compared to the pattern of zebrin-positive and zebrin-negative bands in material double-stained for zebrin II and for different anterograde tracers injected in subnuclei of the inferior olive, or retrograde tracers injected in the cerebellar and vestibular target nuclei of the Purkinje cells of the vermis. Projection zones A1,AX,X,B,CX in the vermis and A2 (accessory A zone) and C2 in the hemisphere were defined by their efferent corticonuclear and their afferent climbing fiber connections, and were found to share the same topographical framework with the zebrin pattern. Introduction There are words that stick in your mind. Among our 1994), the low affinity nerve growth factor receptor favorites are Sanford L. Palay’s statement on synaptic protein (Dusart et al., 1994), protein kinase C delta vesicles, which “like chocolates, come in a variety of (Chen & Hillman, 1993) the glutamate transporter shapes and sizes, and are stuffed with different kind EAAT4 (Dehnes et al., 1998) and the metabotropic of fillings’’ (1967) and his remark on the “chemical id- glutamate receptor mGluR1b (Mateos et al., 2001), to iosyncrasy’’ of non-synaptic monoaminergic cerebellar name only a few.