AP42 Chapter 9 Reference
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Liquid Ammonium Polyphosphate As a Carrier of Iron and Zinc
EVALUATION OF LIQUID AMMONIUM POLYPHOSPHATE AS A CARRIER OF IRON AND ZINC by ; V<P RATMUNDO RALLAN GANIRON B. S. A., University of the Philippines, 1961 A MASTER'S THESIS submitted in partial fulfillment of the requirement for the degree MASTER OF SCIENCE Department of Agronomy KANSAS STATE UNIVERSITY Manhattan, Kansas 1966 Approved by: Jor Professor LP ZGGf it Tj( £/<?7 TABLE OF CONTENTS INTRODUCTION 1 REVIEW OF LITERATURE 3 Iron . 3 Zinc * MATERIALS AND METHODS 13 Fertiliser Materials 13 Field Experiments H Soil Sampling 20 Laaf Sampling 22 Chemical Analysis 22 (a) Zinc 22 (b) Iron 24 (c) Phosphorua 25 (d) Potassium •« 27 (a) Organic Matter 27 (f) pH 27 (g) Protain 27 RESULTS AND DISCUSSIONS 26 SUMMARY AND CONCLUSIONS 4$ ACKNOWLEDGMENT. 51 LITERATURE CITED 52 INTRODUCTION Increasing attention has baan focused to the need of fertilising with micronutrient elements to achieve maximum production and optimum quality of farm crops. The supply of these elements in the soil has become limited in some areas as a result of the introduction of high yielding varieties, better and more intensive cropping practices, and increased use of high analysis fertilisers. In some cases, the ob- served deficiencies of these micronutrients have been man- made due to addition of interfering elements for other pur- poses. For instance there is the copper induced iron chlorosis, copper being introduced as agricultural sprays and the phosphorus induced iron and zinc deficiencies, phos- phorus being introduced to meet the phosphorus requirement of farm crops. How to minimise such interactions and the problems of keeping these micronutrient elements available to the plants when applied to the soil have been the sub- ject of intensive researches. -

Effects of Different Sources of Fertilizer Nitrogen on Growth and Nutrition of Western Hemlock Seedlings
Effects of Different Sources U.S. Department of Agriculture Forest Service Pacific Northwest Forest of FertiIizer Nitrogen and Range Experiment Station Research Paper PNW-267 on Growth and Nutrition oJ February 1980 Western Hemlock Seedlings ---. --_. ------------------------ , I _J Authors M. A. RADWAN is Principal Plant Physiologist and DEAN S. DeBELL is Principal Silviculturist with the Forest Service, u.S. Department of Agriculture, Pacific Northwest Forest and Range Experiment Station, Forestry Sciences Laboratory, Olympia, Washington. En gl ish Equivalents 1 liter 0.2642 gallon 1 kilogram = 2.2046 pound 1 gram = 0.0353 ounce 1 centimeter = 0.3937 inch 1 kilogram per hectare 1.1206 pounds per acre (9/50C) + 32 = of EFFECTS OF DIFFERENT SOURCES OF FERTILIZER NITROGEN ON GROWTH AND NUTRITION OF WESTERN HEMLOCK Reference Abstract Radwan, M. A. , and Dean S. DeBell. 1980. Effects of different sources of fertilizer nitrogen on growth and nutrition of western hemlock seedlings. USDA For. Servo Res. Pap. PNW-267, 15 p. Pacific Northwest Forest and Range Experiment Station, Portland, Oregon. Twelve different nitrogen (N) fertilizer treatments were tested on potted western hemlock (Tsuga heterophylla (Raf. ) Sarg.) seedlings. Fertilizers affected soil N and pH, and growth and foliar chemical com position of seedlings. Ura plus N-Serve and sulfur-coated urea appear more promising for promoting growth than other fertilizers tested. Results, however, do not explain reported variability in response of hemlock stands to N fertilization. Keywords: Nitrogen fertilizer response, seedling growth, western hemlock, Tsuga heterophylla. RESEARCH SUMMARY Research Paper PNW-267 1980 The following fertilization treatments were applied in the spring to potted, 4-year-old western hemlock (Tsuga heterophylla (Raf. -

United States Patent (19) 11 Patent Number: 5,883,058 Wells Et Al
USOO5883058A United States Patent (19) 11 Patent Number: 5,883,058 Wells et al. (45) Date of Patent: *Mar 16, 1999 54 HIGH LATHER STYLING SHAMPOOS 4,784,801 11/1988 Hoeffkes et al. ....................... 252/554 5,084.212 1/1992 Farris et al. ............................ 252/554 (75) Inventors: Robert Lee Wells, Cincinnati, Ohio; 5,104,642 4/1992 Wells et al. ..... ... 424/47 Jon Robert Behrens, Kobe, Japan 5,120,532 6/1992 Wells et al. ............ ... 424/70 5,310,508 5/1994 Subramanyam et al. ............... 252/549 73) Assignee: The Procter & Gamble Company, 5,391,368 2/1995 Gerstein ............................... 424/70.13 5,514,302 5/1996 Brown ..................................... 252/545 Cincinnati, Ohio 5,580,494 12/1996 Sandhu et al. .......................... 510/125 Notice: The term of this patent shall not extend FOREIGN PATENT DOCUMENTS beyond the expiration date of Pat. No. 5,672.576. 0323715 12/1989 European Pat. Off.. Appl. No.: 520,631 Primary Examiner Paul Lieberman Assistant Examiner Necholas Ogden Filed: Aug. 29, 1995 Attorney, Agent, or Firm Joan B. Tucker; William J. Int. Cl." ................................................ C110 1/83 Winter; Tara M. Rosnell U.S. Cl. .......................... 510/127; 510/119,510/123; 57 ABSTRACT 510/125; 424/70.11; 424/70.24 The present invention relates to hair shampoo compositions Field of Search ..................................... 252/549, 550, which have improved cleansing, lathering, and Styling ben 252/551, 557; 510/119, 123,125, 127; efits=. These Shampoo compositions comprise an alkyl glyc 424/70.24, 70.11 eryl ether Sulfonate Surfactant, a hair Styling polymer, a 56) References Cited non-polar volatile Solvent, and water. -

United States Patent (19) 11 Patent Number: 5,788,915 Blount (45) Date of Patent: Aug
IIIUSOO5788915A United States Patent (19) 11 Patent Number: 5,788,915 Blount (45) Date of Patent: Aug. 4, 1998 54 FLAME RETARDANT COMPOSITIONS 57 ABSTRACT UTILIZING PARTIALLY HYDROLYZED AMNO CONDENSATON COMPOUNDS Flame retardant compositions of this invention are produced by incorporating a partially index (LOI) hydrolyzed amino 76 Inventor: David H. Blount, 6728 Del Cerro condensation composition in a more flammable organic Blvd., San Diego, Calif. 92120 material. The partially hydrolyzed amino condensation com pounds are produced by heating urea or heating urea with (21) Appl. No.: 801,776 other nitrogen containing compounds that will condensate with or react with isocyanic acid and/or cyanic acid thereby 22 Filed: Feb. 14, 1997 producing an amino condensation compound which is then Related U.S. Application Data partially hydrolysis is done by reacting it with a limited amount of water. The partially hydrolyzed amino conden 62) Division of Ser. No. 723,779, Sep. 30, 1996. sation compounds may be used alone or may be mixed with or reacted with carbonization auxiliaries, aldehydes and (51) Int. Cl. ........................ C09K 21A00; C08G 12/12 fillers to produce a partially hydrolyzed amino condensation 52 U.S. Cl. ........................... 252/609; 528/259; 252/601 composition which is incorporated in more flammable 58) Field of Search ..................................... 252/609, 601: organic compositions such as polyurethanes, polyester 528/259 resins. epoxy resins, vinyl resins and other resins. The partially hydrolyzed amino condensation salts of 56 References Cited phosphorus, boron or sulfur containing compounds and the U.S. PATENT DOCUMENTS partially hydrolyzed amino condensation-aldehyde resins 3,900,665 8/1975 Weil ....................................... -

(DAP) Diammonium Phosphate Safety Data Sheet 200 Revision Date:04/30/2015 : Version: 1.0
(DAP) Diammonium phosphate Safety Data Sheet 200 Revision date:04/30/2015 : Version: 1.0 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance Substance name : (DAP) Diammonium phosphate Product code : DAP, DAPFR,DAPOS, DAPLG CAS No. : 7783-28-0 Formula : (NH4)2HPO4 Synonyms : Ammonium phosphate, dibasic / Diammonium hydrogenorthophosphate / Phosphoric acid, diammonium salt / Diammonium hydrogenphosphate / Ammonium phosphate dibasic / Diammonium hydrogen phosphate / Diammonium hydrogen orthophosphate / Phosphoric acid, ammonium salt (1:2) / DIAMMONIUM PHOSPHATE / DAP Product group : Commercial product Other means of identification : DAP, DAPLG 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/preparation : Agricultural chemical No additi onal infor mati on available 1.3. Details of the supplier of the safety data sheet PCS Sales (USA), Inc. 1101 Skokie Blvd. Suite 400 Northbrook, IL 60062 T 800-241-6908 / 847-849-4200 Suite 500 122 1st Avenue South Saskatoon, Saskatchewan Canada S7K7G3 T 800-667-0403 (Canada) / 800-667-3930 (USA) [email protected] - www.PotashCorp.com 1.4. Emergency telephone number Emergency number : 800-424-9300 CHEMTREC SECTION 2: Hazards identification 2.1. Classification of the substance or mixture GHS-US classification Skin Irrit. 2 H315 Eye Irrit. 2B H320 STOT SE 3 H335 Aquatic, Acute 2 H401 04/30/2015 EN (English) SDS Ref.: 200 1/10 (DAP) Diammonium phosphate Safety Data Sheet 200 Full text of H-phrases: see section 16 2.2. Label elements GHS-US labelling Hazard pictograms (GHS-US) : GHS07 Signal word (GHS-US) : Warning Hazard statements (GHS-US) : H315 - Causes skin irritation H320 - Causes eye irritation H335 - May cause respiratory irritation H401 - Toxic to aquatic life. -

SIAM 24, 17-20 April 2007 US/ICCA
SIAM 24, 17-20 April 2007 US/ICCA SIDS INITIAL ASSESSMENT PROFILE CAS Nos. 7722-76-1, 7783-28-0, 68333-79-9, 8011-76-5, 65996-95-4 Phosphate category: Monoammonium phosphate (MAP), Chemical Names Diammonium phosphate (DAP), Ammonium polyphosphate (APP), Single superphosphate (SSP), Triple superphosphate (TSP) MAP: NH4H2PO4 DAP: (NH4)2HPO4 Structural Formula APP: SSP: main components:Ca(H2PO4)2. H2O /CaSO4.H2O TSP: CaHPO4.2H2O SUMMARY CONCLUSIONS OF THE SIAR Category/Analogue Rationale The category consists of monoammonium phosphate (MAP; CAS No. 7722-76-1), diammonium phosphate (DAP; CAS No. 7783-28-0), ammonium polyphosphate (APP; CAS No. 68333-79-9), single superphosphate (SSP; CAS No. 8011-76-5), and triple superphosphate (TSP; CAS No. 65996-95-4). All members of the category are mainly or exclusively used as fertilizer and have one common functional group (phosphate) that equilibrates between several different ionic species - = = -3 [H3PO4, H2PO4 , HPO4 , HPO4 , or PO4 ] depending on the pH of the environment. Thus, chemical reactions for all compounds in this category are similar with the exception of the actual dissociation product, which forms calcium or ammonia along with common phosphate moieties. However, the presence of the ammonium ion will influence the observed toxicity and its data are used to conservatively represent the toxicity of the category members. Under typical environmental conditions, the phosphate would be present as monohydrogen = - - phosphate (HPO4 ) or dihydrogen phosphate (H2PO4 ) with the equilibrium favoring H2PO4 as the acidity of the environment increases. Under these conditions, the proportions of phosphoric acid -3 (H3PO4) and the phosphate anion (PO4 ) would be extremely low. -
![An African Green Revolution, Finding Ways to Boost Productivity on Small Farms {Keijiro Otsuka} [940075759X] (2012).Pdf](https://docslib.b-cdn.net/cover/1924/an-african-green-revolution-finding-ways-to-boost-productivity-on-small-farms-keijiro-otsuka-940075759x-2012-pdf-941924.webp)
An African Green Revolution, Finding Ways to Boost Productivity on Small Farms {Keijiro Otsuka} [940075759X] (2012).Pdf
Keijiro Otsuka Donald F. Larson Editors An African Green Revolution Finding Ways to Boost Productivity on Small Farms An African Green Revolution Keijiro Otsuka • Donald F. Larson Editors An African Green Revolution Finding Ways to Boost Productivity on Small Farms Editors Keijiro Otsuka Donald F. Larson National Graduate Institute The World Bank for Policy Studies Research Group 7-2 2-1 Roppongi 1818 H Street Northwest Minato , Tokyo 106-8677 Washington , DC 20433 Japan USA The fi ndings, interpretations, and conclusions expressed in this work do not necessarily re fl ect the views of The World Bank, its Board of Executive Directors, or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. ISBN 978-94-007-5759-2 ISBN 978-94-007-5760-8 (eBook) DOI 10.1007/978-94-007-5760-8 Springer Dordrecht Heidelberg New York London Library of Congress Control Number: 2012951120 © The International Bank for Reconstruction and Development/The World Bank 2013 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, speci fi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on micro fi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. -
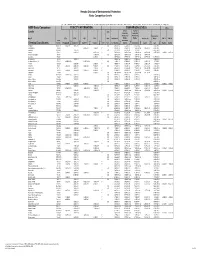
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V -

Prohibited and Restricted Chemical List
School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List PROHIBITED AND RESTRICTED CHEMICAL LIST Introduction After incidents of laboratory chemical contamination at several schools, DCPS, The American Association for the Advancement of Science (AAAS) and DC Fire and Emergency Management Services developed an aggressive program for chemical control to eliminate student and staff exposure to potential hazardous chemicals. Based upon this program, all principals are required to conduct a complete yearly inventory of all chemicals located at each school building to identify for the removal and disposal of any prohibited/banned chemicals. Prohibited chemicals are those that pose an inherent, immediate, and potentially life- threatening risk, injury, or impairment due to toxicity or other chemical properties to students, staff, or other occupants of the school. These chemicals are prohibited from use and/or storage at the school, and the school is prohibited from purchasing or accepting donations of such chemicals. Restricted chemicals are chemicals that are restricted by use and/or quantities. If restricted chemicals are present at the school, each storage location must be addressed in the school's written emergency plan. Also, plan maps must clearly denote the storage locations of these chemicals. Restricted chemicals—demonstration use only are a subclass in the Restricted chemicals list that are limited to instructor demonstration. Students may not participate in handling or preparation of restricted chemicals as part of a demonstration. If Restricted chemicals—demonstration use only are present at the school, each storage location must be addressed in the school's written emergency plan. Section 7: Appendices – October 2009 37 School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List Following is a table of chemicals that are Prohibited—banned, Restricted—academic curriculum use, and Restricted—demonstration use only. -

Fertilizer Use in African Agriculture Lessons Learned and Good Practice Guidelines Public Disclosure Authorized Michael Morris,Valerie A
Public Disclosure Authorized DIRECTIONS IN DEVELOPMENT 39037 Public Disclosure Authorized Agriculture and Rural Development Fertilizer Use in African Agriculture Lessons Learned and Good Practice Guidelines Public Disclosure Authorized Michael Morris,Valerie A. Kelly, Ron J. Kopicki, and Derek Byerlee Public Disclosure Authorized Fertilizer Use in African Agriculture Fertilizer Use in African Agriculture Lessons Learned and Good Practice Guidelines Michael Morris Valerie A. Kelly Ron J. Kopicki Derek Byerlee © 2007 The International Bank for Reconstruction and Development / The World Bank 1818 H Street, NW Washington, DC 20433 Telephone: 202-473-1000 Internet: www.worldbank.org E-mail: [email protected] All rights reserved. 1 2 3 4 :: 10 09 08 07 This volume is a product of the staff of the International Bank for Reconstruction and Development / The World Bank. The findings, interpretations, and conclusions expressed in this volume do not necessarily reflect the views of the Executive Directors of The World Bank or the governments they represent. The World Bank does not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of The World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. Rights and Permissions The material in this publication is copyrighted. Copying and/or transmitting portions or all of this work without permission may be a violation of applicable law. The International Bank for Reconstruction and Development / The World Bank encourages dissemination of its work and will normally grant permission to reproduce portions of the work promptly. -

11-37-0 Liquid Ammonium Polyphosphate Guaranteed Analysis Total Nitrogen (N)
Product Data Sheet 11-37-0 LIQUID AMMONIUM POLYPHOSPHATE GUARANTEED ANALYSIS TOTAL NITROGEN (N) ................................................................................................................................ 11.00% 11.00% Ammoniacal Nitrogen AVAILABLE PHOSPHATE (P2O5) .................................................................................................................37.00% Molybdenum (Mo).........................................................................................................................................0.001% Derived from Ammonium Phosphate. KEEP OUT OF REACH OF CHILDREN tanks and certain fiberglass tanks. Aluminum tanks WARNING can also be used. However, aluminum tanks are not CAUSES SKIN IRRITATION. CAUSES EYE recommended for prolonged storage. IRRITATION. MAY CAUSE RESPIRATORY 2. Pumps: Any pump recommended for use with liquid IRRITATION. fertilizers. Remember the basic rule of "no brass." PRECAUTIONARY STATEMENTS: Avoid breathing fumes / gas / mist / vapors / spray. Wash thoroughly after handling. Use only USES outdoors or in a well-ventilated area. Call a poison center / doctor if 1. Is an excellent source of water soluble, fast acting you feel unwell. Specific treatment see First Aid section on this label. ammonium phosphate with the most desirable ratio of PERSONAL PROTECTIVE EQUIPMENT: Wear protective N:P2O5. gloves / protective clothing / eye protection / face protection. Take off 2. An ideal source of phosphate and nitrogen in complete contaminated clothing and wash before reuse. liquid mixes. FIRST AID: IF ON SKIN Wash with plenty of water. If skin irritation 3. For specific application rates follow the recommendation occurs, get medical advice / attention. IF INHALED Remove person of a qualified individual or institution, such as, but to fresh air and keep comfortable for breathing. IF IN EYES Rinse not limited to, a certified crop advisor, agronomist, cautiously with water for several minutes. Remove contact lenses, if university crop extension publication, or apply present and easy to do. -

Chemical Resistance 100% SOLIDS EPOXY SYSTEMS
Chemical Resistance 100% SOLIDS EPOXY SYSTEMS CHEMICAL 8300 SYSTEM 8200 SYSTEM 8000 SYSTEM OVERKOTE PLUS HD OVERKOTE HD OVERKRETE HD BASED ON ONE YEAR IMMERSION TESTING –––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––– Acetic Acid (0-15%) G II Acetonitrile LLG L Continuous Immersion Acetone (0-20%) LLL Acetone (20-30%) Suitable for continuous immersion in that chemical (based on LLG Acetone (30-50%) L G I ONE YEAR testing) to assure unlimited service life. Acetone (50-100%) G II Acrylamide (0-50%) LLL G Short-Term Exposure Adipic Acid Solution LLL Alcohol, Isopropyl LLL Suitable for short-term exposure to that chemical such as Alcohol, Ethyl LLG secondary containment (72 hours) or splash and spill Alcohol, Methyl LLI (immediate clean-up). Allyl Chloride LLI Allylamine (0-20%) L L I Allylamine (20-30%) L G I I Not Suitable Allylamine (30-50%) GGI Not suitable for any exposure to that chemical. Aluminum Bromide LL– Aluminum Chloride L L – Aluminum Fluoride (0-25%) L L – This chart shows chemical resistance of our various Aluminum Hydroxide LLL 1 topping materials (90 mils – ⁄4"). These ratings are based on Aluminum Iodide LL– temperatures being ambient. At higher temperatures, chemical Aluminum Nitrate LL– resistance may be effected. When chemical exposure is Aluminum Sodium Chloride L L – minimal to non-existent, a 9000 System–FlorClad™ HD or Aluminum Sulfate LLL 4600 System– BriteCast™ HD may be used. Alums L L L 2-Aminoethoxyethanol Resistance data is listed with the assumption that the material GGG has properly cured for at least four days, at recommended Ammonia – Wet L L – temperatures, prior to any chemical exposure.