A 17-Year-Old Baseball Player with Right Hand Weakness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia
brain sciences Review Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia Timothy Fullam and Jeffrey Statland * Department of Neurology, University of Kansas Medical Center, Kansas, KS 66160, USA; [email protected] * Correspondence: [email protected] Abstract: Following the exclusion of potentially reversible causes, the differential for those patients presenting with a predominant upper motor neuron syndrome includes primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), or upper motor neuron dominant ALS (UMNdALS). Differentiation of these disorders in the early phases of disease remains challenging. While no single clinical or diagnostic tests is specific, there are several developing biomarkers and neuroimaging technologies which may help distinguish PLS from HSP and UMNdALS. Recent consensus diagnostic criteria and use of evolving technologies will allow more precise delineation of PLS from other upper motor neuron disorders and aid in the targeting of potentially disease-modifying therapeutics. Keywords: primary lateral sclerosis; amyotrophic lateral sclerosis; hereditary spastic paraplegia Citation: Fullam, T.; Statland, J. Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper 1. Introduction Motor Neuron Dominant Jean-Martin Charcot (1825–1893) and Wilhelm Erb (1840–1921) are credited with first Amyotrophic Lateral Sclerosis, and describing a distinct clinical syndrome of upper motor neuron (UMN) tract degeneration in Hereditary Spastic Paraplegia. Brain isolation with symptoms including spasticity, hyperreflexia, and mild weakness [1,2]. Many Sci. 2021, 11, 611. https:// of the earliest described cases included cases of hereditary spastic paraplegia, amyotrophic doi.org/10.3390/brainsci11050611 lateral sclerosis, and underrecognized structural, infectious, or inflammatory etiologies for upper motor neuron dysfunction which have since become routinely diagnosed with the Academic Editors: P. -

Intrinsic Hand Muscles of the Japanese Monkey, Macaca Fuscata
Anthropol.Sci. 102(Suppl.), 85-95,1994 Intrinsic Hand Muscles of the Japanese Monkey, Macaca fuscata TOSHIHIKO HOMMA AND TATSUO SAKAI Department of Anatomy, School of Medicine, Juntendo University, Hongo 2-1-1, Bunkyo-ku, Tokyo 113, Japan Received December 24, 1993 •ôGH•ô Abstract•ôGS•ô Anatomy of the intrinsic hand muscles in the Japanese monkeys was studied with an improved method to dissect the muscles and nerves in water after removal of the skeletal framework. The thenar eminence contained four muscles, namely m. abductor pollicis brevis, m. opponens pollicis, m, flexor pollicis brevis, and m. adductor pollicis. The hypothenar eminence contained four muscles, namely m. palmaris brevis, m. abductor digiti minimi, m. flexor digiti minimi brevis, and m. opponens digiti minimi. Majority of the thenar muscles are fused more or less with each other, so that clear separation of these muscles was difficult. The lumbrical muscles arose from the palmar parts of four main tendons of the deep flexor muscle to the second, third, fourth, and fifth digits. The mm, contrahentes arose mainly from a medial tendinous septum attached on the palmar surface to the third metacarpus, and included three muscles destined to the second, fourth and fifth digits. The interossei were found on the radial side of the second, third, fourth and fifth finger as well as on the ulnar side of the second, third and fourth finger. The intrinsic hand muscles in the Japanese monkey received innervation either from the median or the ulnar nerve. Branching pattern of these nerves to the individual muscles was fundamentally similar to those in man except for the fact that the median and the ulnar nerve in the Japanese monkey do not communi cateto make a loop in the thenar muscles. -

Inherited Neuropathies
407 Inherited Neuropathies Vera Fridman, MD1 M. M. Reilly, MD, FRCP, FRCPI2 1 Department of Neurology, Neuromuscular Diagnostic Center, Address for correspondence Vera Fridman, MD, Neuromuscular Massachusetts General Hospital, Boston, Massachusetts Diagnostic Center, Massachusetts General Hospital, Boston, 2 MRC Centre for Neuromuscular Diseases, UCL Institute of Neurology Massachusetts, 165 Cambridge St. Boston, MA 02114 and The National Hospital for Neurology and Neurosurgery, Queen (e-mail: [email protected]). Square, London, United Kingdom Semin Neurol 2015;35:407–423. Abstract Hereditary neuropathies (HNs) are among the most common inherited neurologic Keywords disorders and are diverse both clinically and genetically. Recent genetic advances have ► hereditary contributed to a rapid expansion of identifiable causes of HN and have broadened the neuropathy phenotypic spectrum associated with many of the causative mutations. The underlying ► Charcot-Marie-Tooth molecular pathways of disease have also been better delineated, leading to the promise disease for potential treatments. This chapter reviews the clinical and biological aspects of the ► hereditary sensory common causes of HN and addresses the challenges of approaching the diagnostic and motor workup of these conditions in a rapidly evolving genetic landscape. neuropathy ► hereditary sensory and autonomic neuropathy Hereditary neuropathies (HN) are among the most common Select forms of HN also involve cranial nerves and respiratory inherited neurologic diseases, with a prevalence of 1 in 2,500 function. Nevertheless, in the majority of patients with HN individuals.1,2 They encompass a clinically heterogeneous set there is no shortening of life expectancy. of disorders and vary greatly in severity, spanning a spectrum Historically, hereditary neuropathies have been classified from mildly symptomatic forms to those resulting in severe based on the primary site of nerve pathology (myelin vs. -

Neuromuscular Ultrasound in Amyotrophic Lateral Sclerosis Jung Im Seok Department of Neurology, School of Medicine, Catholic University of Daegu, Daegu, Korea
REVIEW ARTICLE pISSN 2635-425X eISSN 2635-4357 JNN https://doi.org/10.31728/jnn.2019.00045 Neuromuscular Ultrasound in Amyotrophic Lateral Sclerosis Jung Im Seok Department of Neurology, School of Medicine, Catholic University of Daegu, Daegu, Korea Ultrasound is a painless and one of the least invasive methods of medical diag- Received: April 16, 2019 nostic testing, which enables visualization of the anatomy of nerves as well as Revised: April 29, 2019 surrounding structures. Over the past decades, researchers have investigated the Accepted: April 30, 2019 ultrasonographic changes that occur in the nerves and muscles of patients with Address for correspondence: motor neuron disease. The current article reviews the sonographic findings that Jung Im Seok are helpful in the diagnosis of amyotrophic lateral sclerosis. The utility of ultra- Department of Neurology, sound in the assessment of respiratory function has also been described. School of Medicine, Catholic J Neurosonol Neuroimag 2019;11(1):73-77 University of Daegu, 33 Duryu- gongwon-ro 17-gil, Nam-gu, Key Words: Ultrasonography; Amyotrophic lateral sclerosis; Diagnosis; Respira- Daegu 42472, Korea Tel: +82-53-650-3440 tion Fax: +82-53-654-9786 E-mail: ji-helpgod@hanmail. net INTRODUCTION upper and lower motor neurons. The disease course is characterized by progressive weakness and muscle Electrodiagnosis (EDX) has traditionally been used atrophy, leading to disability and eventually death. The as the method of choice for evaluation of peripheral diagnosis of ALS is challenging, because of the absence nerves. However, EDX has two key limitations, namely of a diagnostic marker and variability in presentation. the inability to provide anatomic details and patient Although EDX remains the method of choice in confir- discomfort. -

One-Off Sessions 一般演題ポスター 脳波一般・脳電位分布
50th Memorial Annual Meeting of Japanese Society of Clinical Neurophysiology (JSCN) One-off sessions 院機構宇多野病院 脳神経内科, 4.京都大学大学院医学研究 科 てんかん・運動異常生理学講座) 一般演題ポスター 一般演題ポスター 脳波一般・脳電位分布 [P1-9] 過呼吸賦活が脳波検査中の睡眠深度に与える影響 ○秋田萌, 真崎桂, 持田智之, 山田はる香, 田島将太郎, 荻澤恵 [P1-1] 小児脳波検査時における薬物鎮静の睡眠賦活有効性と 美, 矢冨裕, 代田悠一郎 (東京大学医学部附属病院 検査 安全性について 部) ○ 吉兼綾美, 石原尚子, 古川源, 石丸聡一郎, 三宅未紗, 河村吉 [P1-10] Bickerstaff型脳幹脳炎における上行性網様体賦活系 紀, 吉川哲史 (藤田医科大学 医学部 小児科) の障害に伴う脳波変化 [P1-2] うつ病患者の安静脳波における前頭部の機能的・因果 ○吉村元1, 十河正弥2, 石井淳子1, 比谷里美1, 乾涼磨1, 中澤 的結合性指標と経頭蓋磁気刺激療法の治療効果との関 晋作1, 木村正夢嶺1, 黒田健仁1, 角替麻里絵1, 石山浩之1, 連 前川嵩太1, 村上泰隆1, 藤原悟1, 尾原信行1, 川本未知1, 幸原 ○ 1,2 2 2 2,3 2 高野万由子 , 和田真孝 , 李雪梅 , 中西智也 , 本多栞 , 伸夫1 (1.神戸市立医療センター中央市民病院 脳神経内 2 2 2 2 2 新井脩泰 , 三村悠 , 宮崎貴浩 , 中島振一郎 , 三村將 , 野田賀 科, 2.神戸大学大学院医学研究科 脳神経内科学分野) 2 大 (1.帝人ファーマ株式会社 医療技術研究所, 2.慶應義塾 [P1-11] 新型コロナウィルス感染患者における脳波検査の実 大学 医学部 精神・神経科学教室, 3.東京大学大学院 総 際 合文化研究科 身体運動科学研究室) ○真崎桂1, 持田智之1, 小口絢子2, 山田はる香1, 田島将太郎1, [P1-3] 脳波異常を呈した聴神経鞘腫に伴う水頭症の1例 秋田萌1, 荻澤恵美1, 矢冨裕1, 代田悠一郎1,2 (1.東大病院 ○ 1 1 2 1 2 山岡美奈子 , 岩佐直毅 , 中瀬健太 , 眞野智生 , 中瀬裕之 , 検査部, 2.東大病院 脳神経内科) 1 杉江和馬 (1.奈良県立医科大学 脳神経内科学, 2.奈良県立 [P1-12] 慢性シンナー中毒の若年男性に発症したてんかん発 医科大学 脳神経外科) 作:脳波所見の変化 [P1-4] 発達障害特性をもつ就学前幼児における発作性脳波異 ○下園孝治1, 加藤志都2, 日野恵理子2, 毛利祐子2, 松堂早矢 常の検討 加2, 西田紬2 (1.健和会大手町病院 内科, 2.健和会大手町 ○ 1 2 1 3 伊予田邦昭 , 三谷納 , 荻野竜也 , 三宅進 (1.福山市こど 病院 生理検査室) も発達支援センター, 2.福山市民病院 小児科, 3.重症心身障 [P1-13] 脳波パワー値解析による NMDA受容体脳炎と単純ヘ 害児者施設 ときわ呉) ルペス脳炎の鑑別指標の検討 [P1-5] 進行性ミオクローヌスてんかんにおけるペランパネル ○溝口知孝, 原誠, 田崎健太, 大下菜月, 名取直俊, 廣瀬聡, の脳波への影響:後頭部優位律動の検討 横田優樹, 秋本高義, 二宮智子, 石原正樹, 森田昭彦, 中嶋秀 ○ 1 2 3 3 2 坂東宏樹 , 戸島麻耶 , 松橋眞生 , 宇佐美清英 , 高橋良輔 , 人 (日本大学 医学部 内科学系 -

Split Hand Syndrome in a Family with GARS-Associated Axonal Neuropathy
JCN Open Access LETTER TO THE EDITOR pISSN 1738-6586 / eISSN 2005-5013 / J Clin Neurol 2019 Split Hand Syndrome in a Family with GARS-Associated Axonal Neuropathy You-Ri Kanga* Dear Editor, Kyung-Wook Kanga* A 21-year-old man presented with weakness in both hands that had been present for 2 Tai-Seung Nama,b years. A neurological examination revealed bilaterally symmetrical distal motor weakness Jae-Hwan Ima (MRC grade 4+) and atrophy in both hands with no sensory loss. The atrophy was confined Sang-Hoon Kima to the lateral side of the hands including the abductor pollicis brevis (APB) and first dorsal Seung-Jin Leec interosseous (FDI) muscles, sparing the hypothenar muscles including abductor digiti mini- aDepartments of Neurology and mi (ADM) on the medial side of the hands (Fig. 1A and B), thus suggesting dissociated atro- cRadiology, Chonnam National University phy of the intrinsic hand muscles. Nerve conduction study (NCS) indicated reduced com- Hospital, Gwangju, Korea bDepartment of Neurology, pound muscle action potential (CMAP) amplitudes when stimulating the median nerve, Chonnam National University but unremarkable findings in the ulnar nerve (Fig. 1E). Electromyography revealed chronic Medical School, Gwangju, Korea neurogenic denervation in the APB and FDI, but not in the other C8–T1 innervated mus- cles including the ADM and flexor carpi ulnaris. Cervical magnetic resonance imaging (MRI) revealed Arnold-Chiari type I malformation with focal syringomyelia at the C6–C7 level. There was no radiological evidence of Hirayama disease. The 51-year-old mother of the patient had experienced chronic muscle weakness in both hands since her early twenties (Fig. -

The Muscles That Act on the Upper Limb Fall Into Four Groups
MUSCLES OF THE APPENDICULAR SKELETON UPPER LIMB The muscles that act on the upper limb fall into four groups: those that stabilize the pectoral girdle, those that move the arm, those that move the forearm, and those that move the wrist, hand, and fingers. Muscles Stabilizing Pectoral Girdle (Marieb / Hoehn – Chapter 10; Pgs. 346 – 349; Figure 1) MUSCLE: ORIGIN: INSERTION: INNERVATION: ACTION: ANTERIOR THORAX: anterior surface coracoid process protracts & depresses Pectoralis minor* pectoral nerves of ribs 3 – 5 of scapula scapula medial border rotates scapula Serratus anterior* ribs 1 – 8 long thoracic nerve of scapula laterally inferior surface stabilizes / depresses Subclavius* rib 1 --------------- of clavicle pectoral girdle POSTERIOR THORAX: occipital bone / acromion / spine of stabilizes / elevates / accessory nerve Trapezius* spinous processes scapula; lateral third retracts / rotates (cranial nerve XI) of C7 – T12 of clavicle scapula transverse processes upper medial border elevates / adducts Levator scapulae* dorsal scapular nerve of C1 – C4 of scapula scapula Rhomboids* spinous processes medial border adducts / rotates dorsal scapular nerve (major / minor) of C7 – T5 of scapula scapula * Need to be familiar with on both ADAM and the human cadaver Figure 1: Muscles stabilizing pectoral girdle, posterior and anterior views 2 BI 334 – Advanced Human Anatomy and Physiology Western Oregon University Muscles Moving Arm (Marieb / Hoehn – Chapter 10; Pgs. 350 – 352; Figure 2) MUSCLE: ORIGIN: INSERTION: INNERVATION: ACTION: intertubercular -
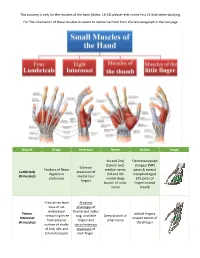
This Sumarry Is Only for the Muscles of the Hand (Slides: 14-33) Please Refer to the First 13 Slide When Studying
This sumarry is only for the muscles of the hand (slides: 14-33) please refer to the first 13 slide when studying. For The innervation of these muscles its easier to memorize them from the last paragraph in the last page Muscle Origin Insertion Nerve Action Image 1st and 2nd, Flexmetacarpoph (lateral two) alangeal (MP) Extensor Tendons of flexor median nerve; joints & extend Lumbricals expansion of Digitorum 3rd and 4th interphalangeal (4 muscles) medial four profundus medial deep (IP) joints of fingers branch of ulnar fingers except nerve thumb -First arises from Proximal base of 1st phalanges of metacarpal thumb and index, Palmar adduct fingers - remaining three ring, and little Deep branch of Interossei toward center of from anterior fingers and ulnar nerve (4 muscles) third finger surface of shafts dorsal extensor of 2nd, 4th, and expansion of 5th metacarpals each finger . Proximal phalanges of index, middle, and ring fingers Contiguous sides abduct fingers Dorsal Interossei and dorsal Deep branch of of shafts of from center of (4 muscles) extensor ulnar nerve metacarpal bones third finger expansion (1st:index\ 2nd,3rd:middle \ 4th:ring) Both palmar and dorsal: -Flex metacarpophalangeal joints -Extend interphalangeal joints Simultaneous flexion at the metacarpophalangeal joints and extension at the interphalangeal joints of a digit are essential for the fine movements of writing, drawing, threading a needle, etc. The Lumbricals and interossei have long been accepted as not only primary agents in flexing the metacarpophalangeal joints -

ISPUB.COM Volume 9 Number 2
The Internet Journal of Surgery ISPUB.COM Volume 9 Number 2 An Anomalous Palmaris Brevis Muscle and its Clinical Implications S Das, S Paul Citation S Das, S Paul. An Anomalous Palmaris Brevis Muscle and its Clinical Implications. The Internet Journal of Surgery. 2006 Volume 9 Number 2. Abstract The palmaris brevis (PB) muscle is a small, thin, subcutaneous muscle of the palm. The palmaris brevis muscle is important muscle contributing to the grip of the hand. In the present case, during routine cadaveric dissection, we detected a PB muscle which was not subcutaneous but existed as a muscle deep to the dermis of the skin of the palm. The PB muscle stretched as a horizontal band over the hypothenar muscles of the palm. The anomalous location of the PB may result in compression of neurovascular structures on the ulnar side of the palm. The awareness of such anomalous muscle which is not subcutaneous, may be clinical important for surgeons operating on the hand and clinicians diagnosing compressive symptoms. INTRODUCTION Figure 1 The PB muscle is a thin subcutaneous muscle. The PB arises Figure 1: Photograph of dissected right palm showing from the flexor retinaculum and the medial border of the central part of the palmar aponeurosis and attached to the dermis of the skin on the ulnar side of the palm. 1 The PB is innervated by the ulnar nerve and the main action of the muscle makes the palmar grip more secure. 1 The clinical importance of the muscle lies in the fact, that it lies above the ulnar artery and the ulnar nerve in the palm. -

Dissociated Leg Muscle Atrophy in Amyotrophic Lateral
www.nature.com/scientificreports OPEN Dissociated leg muscle atrophy in amyotrophic lateral sclerosis/ motor neuron disease: the ‘split‑leg’ sign Young Gi Min1,4, Seok‑Jin Choi2,4, Yoon‑Ho Hong3, Sung‑Min Kim1, Je‑Young Shin1 & Jung‑Joon Sung1* Disproportionate muscle atrophy is a distinct phenomenon in amyotrophic lateral sclerosis (ALS); however, preferentially afected leg muscles remain unknown. We aimed to identify this split‑leg phenomenon in ALS and determine its pathophysiology. Patients with ALS (n = 143), progressive muscular atrophy (PMA, n = 36), and age‑matched healthy controls (HC, n = 53) were retrospectively identifed from our motor neuron disease registry. We analyzed their disease duration, onset region, ALS Functional Rating Scale‑Revised Scores, and results of neurological examination. Compound muscle action potential (CMAP) of the extensor digitorum brevis (EDB), abductor hallucis (AH), and tibialis anterior (TA) were reviewed. Defned by CMAPEDB/CMAPAH (SIEDB) and CMAPTA/CMAPAH (SITA), respectively, the values of split‑leg indices (SI) were compared between these groups. SIEDB was signifcantly reduced in ALS (p < 0.0001) and PMA (p < 0.0001) compared to the healthy controls (HCs). SITA reduction was more prominent in PMA (p < 0.05 vs. ALS, p < 0.01 vs. HC), but was not signifcant in ALS compared to the HCs. SI was found to be signifcantly decreased with clinical lower motor neuron signs (SIEDB), while was rather increased with clinical upper motor neuron signs (SITA). Compared to the AH, TA and EDB are more severely afected in ALS and PMA patients. Our fndings help to elucidate the pathophysiology of split‑leg phenomenon. -

Compressive Mononeuropathies of the Upper Extremity in Chronic Paraplegia
ParapkgW 29 (1991) 17-24 © 1991 International Medical Society of Paraplegia Paraplegia Compressive Mononeuropathies of the Upper Extremity in Chronic Paraplegia G. Davidoff, MD, R. Werner, MD, W. Waring, MD Department of Physical Medicine and Rehabilitation, University of Michigan Medical Center, and Rehabilitation Medicine Service, Veterans Affairs Medical Center, Ann Arbor, Michigan, USA. Summary Controversy exists with regard to the actual prevalence of compressive mononeuropathies at the wrist which may occur following chronic paraplegia. Thirty one chronic paraplegics, with a mean age of 37'9 years (range 20-68 years), and mean time since injury of 9'7 years (range 1-28 years), were studied with a comprehensive neurologic and electrodiagnostic (EDX) assessment. No patient had any clinical or EDX evidence of a peripheral polyneuropathy. The diagnosis of a median mononeuropathy at the wrist was determined by the following criteria: (a) prolonged median sensory distal latency > ipsilateral ulnar sensory distal latency 2 0·5 msec; (b) a median mid-palmar sensory latency> ipsilateral ulnar mid-palmar sensory latency of 2 0'3 msec; or (c) a median motor distal latency 2 l' 7 milliseconds as compared to the ipsilateral ulnar motor distal latency. Ulnar mononeuropathy at the wrist or across the elbow was also characterised. The EDX criteria for a median mononeuropathy at the wrist was met in 55% of subjects (24% of these with bilateral presentations). The location of ulnar mononeuropathies included: two at the superficial sensory branch at the wrist, one at the deep motor branch at the wrist, and three patients with a conduction block across the elbow. -

Split-Hand Phenomenon Quantified by the Motor Unit Number Index For
Neurophysiologie Clinique/Clinical Neurophysiology (2019) 49, 391—404 Disponible en ligne sur ScienceDirect www.sciencedirect.com ORIGINAL ARTICLE Split-hand phenomenon quantified by the motor unit number index for distinguishing cervical spondylotic amyotrophy from amyotrophic lateral sclerosis a,1 b a c,1 Chaojun Zheng , Yu Zhu , Minghao Shao , Dongqing Zhu , d,1 c a,∗ Hong Hu , Kai Qiao , Jianyuan Jiang a Department of Orthopedics, Huashan Hospital, Fudan University, 200040 Shanghai, China b Department of Physical Medicine and Rehabilitation, Upstate Medical University, State University of New York at Syracuse, 10212 Syracuse, NY, USA c Department of Neurology, Huashan Hospital, Fudan University, 200040 Shanghai, China d Department of Emergency, Huashan Hospital, Fudan University, 200040 Shanghai, China Received 14 September 2019; accepted 24 September 2019 Available online 11 October 2019 KEYWORDS Summary Cervical spondylotic Objectives. — To investigate and compare split-hand phenomenon quantified by motor unit num- amyotrophy; ber index (MUNIX) between patients with cervical spondylotic amyotrophy (CSA) and those with Amyotrophic lateral amyotrophic lateral sclerosis (ALS). sclerosis; Methods. — MUNIX was performed on abductor pollicis brevis (APB), abductor digiti minimi (ADM) Split-hand and first dorsal interosseous (FDI) in 46 CSA patients, 39 ALS patients and 41 healthy subjects. phenomenon; Split-hand measurements including split-hand index (SHI = ABP × FDI/ADM), ratio of APB to ADM Motor unit number (AA), ratio of FDI to ADM (FA) were measured by compound muscle action potential (CMAP) and index; MUNIX. Differential diagnosis Results. — There was a significant difference in both AA and SHI measured by two different methods between ALS and CSA patients (P < 0.05).