Isotopes Tell Origin and Operation of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Dole Effect and Its Variations During the Last 130,000 Years As Measured in the Vostok Ice Core
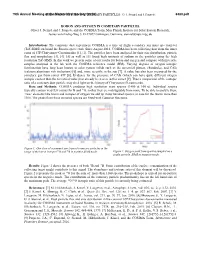
GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 8, NO. 3, PAGES 363-376, SEPTEMBER 1994 The Dole effect and its variations during the last 130,000 years as measured in the Vostok ice core Michael Bender • and Todd Sowers 2 GraduateSchool of Oceanography,University of RhodeIsland, Kingston Laurent Labeyrie Centredes Faibles Radioactivites, Centre National de la RechercheScientifique, Commissariat a L'EnergieAtomique, Gif-sur- Yvette, France Abstract. We review the currentunderstanding of the Dole effect (the observeddifference between the •5180of atmospheric0 2 andthat of seawater)and its causes, extend the record of variationsin theDole effectback to 130kyr before present using data on the •5180 of 0 2obtained from studying the Vostok ice core(Sowers et al., 1993), anddiscuss the significanceof temporalvariations. The Dole effectreflects oxygenisotope fractionation during photosynthesis, respiration, and hydrologic processes (evaporation, precipitation,and evapotranspiration). Our bestprediction of the present-dayDole effect,+20.8 %0,is considerablylower thanthe observedvalue, + 23.5 %0,and we discusspossible causes of this discrepancy. During the past 130 kyr, the Dole effecthas been 0.05 %0lower thanthe present value, on average.The standarddeviation of the Dole effect from the meanhas been only _+0.2 %0,and the Dole effect is nearly unchangedbetween glacial maxima and interglacial periods. The smallvariability in theDole effect suggeststhat relative rates of primaryproduction in theland and marine realms have been relatively constant.Most periodicvariability in the Dole effectis in the precessionband, suggesting that changes in thisglobal biogeochemical term reflects variations in low-latitudeland hydrology and productivity or possiblyvariability in low-latitudeoceanic productivity. Introduction they can potentially be made to do so. Ice core reconstructionsof the concentrationsof bioactivegases in air Our understanding of the response of the biosphere to provide an integratedglobal signal which complementsthe Pleistoceneclimate change is actually quite limited. -

Capabilities of Detecting Medical Isotope Facilities Through Radioxenon Sampling
AN ABSTRACT OF THE THESIS OF Matthew R. MacDougall for the degree of Master of Science in Nuclear Engineering presented on June 23, 2015. Title: Capabilities of Detecting Medical Isotope Facilities through Radioxenon Sampling Abstract approved: ______________________________________________________ Andrew C. Klein Medical Isotopes are a necessity in modern medicine for cancer treatments and medical imaging. In order to ensure that the needs and demands are met for the medical procedures, facilities are put in place to produce these isotopes. There are over 25 different isotopes of interest being produced by more than 35 research reactors across the United States. A key component in medical isotope production is the isotope separation process. During this process, several types of radioactive gases are released that would otherwise not leave the nuclear fuel component. One of these radioactive gases is radioxenon. The release of radioxenon into the environment is of concern to the Comprehensive Test Ban Treaty Organization (CTBTO) as one of the key critical sampling techniques utilized to detect a nuclear detonation is the presence of radioxenon. As more facilities release radioxenon, background levels increase, desensitizing the equipment, and making it more difficult to detect. For this purpose, the detection of a medical isotope facility through the use of radioxenon is an interest to the CTBTO as an attempt to reduce the background levels of radioxenon and ensure that the detonation capabilities remain unaffected. This thesis will investigate the capabilities of detecting these medical isotope facilities through the use of radioxenon detection. Additionally, probabilities of detection will be determined in order to accurately identify these facilities. -

12 Natural Isotopes of Elements Other Than H, C, O
12 NATURAL ISOTOPES OF ELEMENTS OTHER THAN H, C, O In this chapter we are dealing with the less common applications of natural isotopes. Our discussions will be restricted to their origin and isotopic abundances and the main characteristics. Only brief indications are given about possible applications. More details are presented in the other volumes of this series. A few isotopes are mentioned only briefly, as they are of little relevance to water studies. Based on their half-life, the isotopes concerned can be subdivided: 1) stable isotopes of some elements (He, Li, B, N, S, Cl), of which the abundance variations point to certain geochemical and hydrogeological processes, and which can be applied as tracers in the hydrological systems, 2) radioactive isotopes with half-lives exceeding the age of the universe (232Th, 235U, 238U), 3) radioactive isotopes with shorter half-lives, mainly daughter nuclides of the previous catagory of isotopes, 4) radioactive isotopes with shorter half-lives that are of cosmogenic origin, i.e. that are being produced in the atmosphere by interactions of cosmic radiation particles with atmospheric molecules (7Be, 10Be, 26Al, 32Si, 36Cl, 36Ar, 39Ar, 81Kr, 85Kr, 129I) (Lal and Peters, 1967). The isotopes can also be distinguished by their chemical characteristics: 1) the isotopes of noble gases (He, Ar, Kr) play an important role, because of their solubility in water and because of their chemically inert and thus conservative character. Table 12.1 gives the solubility values in water (data from Benson and Krause, 1976); the table also contains the atmospheric concentrations (Andrews, 1992: error in his Eq.4, where Ti/(T1) should read (Ti/T)1); 2) another category consists of the isotopes of elements that are only slightly soluble and have very low concentrations in water under moderate conditions (Be, Al). -

Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T
U.S. Department of the Interior U.S. Geological Survey Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T. B. Coplen', J. A. Hopple', J. K. Bohlke', H. S. Reiser', S. E. Rieder', H. R. o *< A R 1 fi Krouse , K. J. R. Rosman , T. Ding , R. D. Vocke, Jr., K. M. Revesz, A. Lamberty, P. Taylor, and P. De Bievre6 'U.S. Geological Survey, 431 National Center, Reston, Virginia 20192, USA 2The University of Calgary, Calgary, Alberta T2N 1N4, Canada 3Curtin University of Technology, Perth, Western Australia, 6001, Australia Institute of Mineral Deposits, Chinese Academy of Geological Sciences, Beijing, 100037, China National Institute of Standards and Technology, 100 Bureau Drive, Stop 8391, Gaithersburg, Maryland 20899 "institute for Reference Materials and Measurements, Commission of the European Communities Joint Research Centre, B-2440 Geel, Belgium Water-Resources Investigations Report 01-4222 Reston, Virginia 2002 U.S. Department of the Interior GALE A. NORTON, Secretary U.S. Geological Survey Charles G. Groat, Director The use of trade, brand, or product names in this report is for identification purposes only and does not imply endorsement by the U.S. Government. For additional information contact: Chief, Isotope Fractionation Project U.S. Geological Survey Mail Stop 431 - National Center Reston, Virginia 20192 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286 Denver, CO 80225-0286 CONTENTS Abstract...................................................... -
Boron and Oxygen in Cometary Particles: O. J
79th Annual Meeting ofBORON the Meteoritical AND OXYGEN Society IN COMETARY (2016) PARTICLES: O. J. Stenzel and J. Paquette 6380.pdf BORON AND OXYGEN IN COMETARY PARTICLES. Oliver J. Stenzel and J. Paquette and the COSIMA Team, Max Planck Institute for Solar System Research, Justus-von-Liebig-Weg 3, D-37077 Göttingen, Germany, [email protected]. Introduction: The cometary dust experiment COSIMA is a time of flight secondary ion mass spectrometer (ToF-SIMS) on board the Rosetta space craft. Since August 2014, COSIMA has been collecting dust from the inner coma of 67P/Churyumov-Gerasimenko [1], [2]. The particles have been analyzed for their size distribution, particle flux and morphology [3], [4]. [5] as well as [1] found high amounts of sodium in the particles using the high resolution ToF-SIMS. In this work we present some of our results for boron and oxygen and compare with meteorite samples analyzed in the lab with the COSIMA reference model (RM). Varying degrees of oxygen isotopic fractionation have long been known in solar system solids such as the terrestrial planets, chondrules, and CAIs (calcium-aluminum-rich inclusions) [6] and, more recently, in the sun [7]. A value has also been measured for the cometary gas from comet 67P [8]. Evidence for the presence of CAIs (which can have quite different oxygen isotopic content than the terrestrial value) has already been seen in this comet [9]. Thus a comparison of the isotopic ratio of a cometary dust particle may shed light on the history of Churyumov-Gerasimenko. Data and Methods: COSIMA produces high resolution mass spectra (1400 at 100 u). -

Investigation of Nitrogen Cycling Using Stable Nitrogen and Oxygen Isotopes in Narragansett Bay, Ri
University of Rhode Island DigitalCommons@URI Open Access Dissertations 2014 INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI Courtney Elizabeth Schmidt University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/oa_diss Recommended Citation Schmidt, Courtney Elizabeth, "INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI" (2014). Open Access Dissertations. Paper 209. https://digitalcommons.uri.edu/oa_diss/209 This Dissertation is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI BY COURTNEY ELIZABETH SCHMIDT A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN OCEANOGRAPHY UNIVERSITY OF RHODE ISLAND 2014 DOCTOR OF PHILOSOPHY DISSERTATION OF COURTNEY ELIZABETH SCHMIDT APPROVED: Thesis Committee: Major Professor Rebecca Robinson Candace Oviatt Arthur Gold Nassar H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2014 ABSTRACT Estuaries regulate nitrogen (N) fluxes transported from land to the open ocean through uptake and denitrification. In Narragansett Bay, anthropogenic N loading has increased over the last century with evidence for eutrophication in some regions of Narragansett Bay. Increased concerns over eutrophication prompted upgrades at wastewater treatment facilities (WWTFs) to decrease the amount of nitrogen discharged. The upgrade to tertiary treatment – where bioavailable nitrogen is reduced and removed through denitrification – has occurred at multiple facilities throughout Narragansett Bay’s watershed. -

(A) Two Stable Isotopes of Lithium and Have Respective Abundances of And
NCERT Solution for Class 12 Physics :Chapter 13 Nuclei Q.13.1 (a) Two stable isotopes of lithium and have respective abundances of and . These isotopes have masses and , respectively. Find the atomic mass of lithium. Answer: Mass of the two stable isotopes and their respective abundances are and and and . m=6.940934 u Q. 13.1(b) Boron has two stable isotopes, and . Their respective masses are and , and the atomic mass of boron is 10.811 u. Find the abundances of and . Answer: The atomic mass of boron is 10.811 u Mass of the two stable isotopes are and respectively Let the two isotopes have abundances x% and (100-x)% Aakash Institute Therefore the abundance of is 19.89% and that of is 80.11% Q. 13.2 The three stable isotopes of neon: and have respective abundances of , and . The atomic masses of the three isotopes are respectively. Obtain the average atomic mass of neon. Answer: The atomic masses of the three isotopes are 19.99 u(m 1 ), 20.99 u(m 2 ) and 21.99u(m 3 ) Their respective abundances are 90.51%(p 1 ), 0.27%(p 2 ) and 9.22%(p 3 ) The average atomic mass of neon is 20.1771 u. Q. 13.3 Obtain the binding energy( in MeV ) of a nitrogen nucleus , given m Answer: m n = 1.00866 u m p = 1.00727 u Atomic mass of Nitrogen m= 14.00307 u Mass defect m=7 m n +7 m p - m m=7Aakash 1.00866+7 1.00727 - 14.00307 Institute m=0.10844 Now 1u is equivalent to 931.5 MeV E b =0.10844 931.5 E b =101.01186 MeV Therefore binding energy of a Nitrogen nucleus is 101.01186 MeV. -

Bibliographie Sur Le Plutonium Et M Composés. •Ibkography On
DIRIAN J.. CHOQUET J. Rapport CEA n» 796 Bibliographie sur le plutonium et m composés. Sommairo. — Compilation de références bibliographiques sur le plutonium et s»s princi- paux composés de 1942 à fin 1957. 1958 82 pages DIRIAN J.. CHOQUET J. Report CEA n» 796 •ibKography on pMommn and its j- — Collection of bibliographical references on plutonium and its principal com- pounds from 1942 to end of 1957. 1958 82 pages PRÉSIDENCE OU CONSEIL COMMISSARIAT A L'ÉNERGIE ATOMIQUE BIBLIOGRAPHIE SUR LE PLUTONIUM ET SES COMPOSÉS par Mme J. DIRIAN et Mlle J CHOQUET Rapport CEA n° 796 CENTRE D'ÉTUDES NUCLEAIRES DE SACLAY SERVICE DE DOCUMENTATION Boite portai* n° 2 - Gif-sur-Yv«H* (S.-^-O.) COMMISSARIAT A L'EEERGIE ATOMIQUE Service de Documentati«n BIBLIOGRAPHIE SUR LE PLUTONIUM ET SES COMPOSES 1 ère Partie par Mme DIRIAN et Mlle CHOQUET Rapport CE.A. N° 796 - Janvier 1953 - ..- I - La présente bibliographie, sur le plutonium cotiporte deux types de références : 1° les rapports déclassés américains U S A E C canadiens A E C L anglais . U. £ A E A 2# les articles parus dans les différentes revues scientifiques, r Elle est divisée en 7 chapitres. 1 - Préparation. Extraction. Purification . 1 - 187 2 - Analyse Dosage 18R - 254 3 - Propriétés physiques 255 - 321 4 - Propriétés nucléaires. Isotopes. a) Le plutonium dans les piles 322 - 349 1B) Etude* de plusieurs isotopes 550 - 428 c) Ilutv>nium-232 h 238 429 - 434 d) Plutonium-239 435 - 534 e) Plutonium-240 535 - 583 5 - Propriétés chimiques 584 - 655 6 - Métallurgie. Alliages 656 - 701 7 - Principaux composés. -

Mass Fraction and the Isotopic Anomalies of Xenon and Krypton in Ordinary Chondrites
Scholars' Mine Masters Theses Student Theses and Dissertations 1971 Mass fraction and the isotopic anomalies of xenon and krypton in ordinary chondrites Edward W. Hennecke Follow this and additional works at: https://scholarsmine.mst.edu/masters_theses Part of the Chemistry Commons Department: Recommended Citation Hennecke, Edward W., "Mass fraction and the isotopic anomalies of xenon and krypton in ordinary chondrites" (1971). Masters Theses. 5453. https://scholarsmine.mst.edu/masters_theses/5453 This thesis is brought to you by Scholars' Mine, a service of the Missouri S&T Library and Learning Resources. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. MASS FRACTIONATION AND THE ISOTOPIC ANOMALIES OF XENON AND KRYPTON IN ORDINARY CHONDRITES BY EDWARD WILLIAM HENNECKE, 1945- A THESIS Presented to the Faculty of the Graduate School of the UNIVERSITY OF MISSOURI-ROLLA In Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE IN CHEMISTRY 1971 T2572 51 pages by Approved ~ (!.{ 1.94250 ii ABSTRACT The abundance and isotopic composition of all noble gases are reported in the Wellman chondrite, and the abundance and isotopic composition of xenon and krypton are reported in the gases released by stepwise heating of the Tell and Scurry chondrites. Major changes in the isotopic composition of xenon result from the presence of radio genic Xel29 and from isotopic mass fractionation. The isotopic com position of trapped krypton in the different temperature fractions of the Tell and Scurry chondrites also shows the effect of isotopic fractiona tion, and there is a covariance in the isotopic composition of xenon with krypton in the manner expected from mass dependent fractiona tion. -

The New Nuclear Forensics: Analysis of Nuclear Material for Security
THE NEW NUCLEAR FORENSICS Analysis of Nuclear Materials for Security Purposes edited by vitaly fedchenko The New Nuclear Forensics Analysis of Nuclear Materials for Security Purposes STOCKHOLM INTERNATIONAL PEACE RESEARCH INSTITUTE SIPRI is an independent international institute dedicated to research into conflict, armaments, arms control and disarmament. Established in 1966, SIPRI provides data, analysis and recommendations, based on open sources, to policymakers, researchers, media and the interested public. The Governing Board is not responsible for the views expressed in the publications of the Institute. GOVERNING BOARD Sven-Olof Petersson, Chairman (Sweden) Dr Dewi Fortuna Anwar (Indonesia) Dr Vladimir Baranovsky (Russia) Ambassador Lakhdar Brahimi (Algeria) Jayantha Dhanapala (Sri Lanka) Ambassador Wolfgang Ischinger (Germany) Professor Mary Kaldor (United Kingdom) The Director DIRECTOR Dr Ian Anthony (United Kingdom) Signalistgatan 9 SE-169 70 Solna, Sweden Telephone: +46 8 655 97 00 Fax: +46 8 655 97 33 Email: [email protected] Internet: www.sipri.org The New Nuclear Forensics Analysis of Nuclear Materials for Security Purposes EDITED BY VITALY FEDCHENKO OXFORD UNIVERSITY PRESS 2015 1 Great Clarendon Street, Oxford OX2 6DP, United Kingdom Oxford University Press is a department of the University of Oxford. It furthers the University’s objective of excellence in research, scholarship, and education by publishing worldwide. Oxford is a registered trade mark of Oxford University Press in the UK and in certain other countries © SIPRI 2015 The moral rights of the authors have been asserted All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of SIPRI, or as expressly permitted by law, or under terms agreed with the appropriate reprographics rights organizations. -

Chapter 16 Nuclear Chemistry
Chapter 16 275 Chapter 16 Nuclear Chemistry Review Skills 16.1 The Nucleus and Radioactivity Nuclear Stability Types of Radioactive Emissions Nuclear Reactions and Nuclear Equations Rates of Radioactive Decay Radioactive Decay Series The Effect of Radiation on the Body 16.2 Uses of Radioactive Substances Medical Uses Carbon-14 Dating Other Uses for Radioactive Nuclides 16.3 Nuclear Energy Nuclear Fission and Electric Power Plants Nuclear Fusion and the Sun Special Topic 16.1: A New Treatment for Brain Cancer Special Topic 16.2: The Origin of the Elements Chapter Glossary Internet: Glossary Quiz Chapter Objectives Review Questions Key Ideas Chapter Problems 276 Study Guide for An Introduction to Chemistry Section Goals and Introductions Section 16.1 The Nucleus and Radioactivity Goals To introduce the new terms nucleon, nucleon number, and nuclide. To show the symbolism used to represent nuclides. To explain why some nuclei are stable and others not. To provide you with a way of predicting nuclear stability. To describe the different types of radioactive decay. To show how nuclear reactions are different from chemical reactions. To show how nuclear equations are different from chemical equations. To show how the rates of radioactive decay can be described with half-life. To explain why short-lived radioactive atoms are in nature. To describe how radiation affects our bodies.. This section provides the basic information that you need to understand radioactive decay. It will also help you understand the many uses of radioactive atoms, including how they are used in medicine and in electricity generation. Section 16.2 Uses of Radioactive Substances Goal: To describe many of the uses of radioactive atoms, including medical uses, archaeological dating, smoke detectors, and food irradiation. -

All About Elements: Neon
All About Elements: Neon 1 Ward’s All About Elements Series Building Real-World Connections to the Building Blocks of Chemistry PERIODIC TABLE OF THE ELEMENTS GROUP 1/IA 18/VIIIA 1 2 H KEY He Atomic Number 1.01 2/IIA 35 13/IIIA 14/IVA 15/VA 16/VIA 17/VIIA 4.00 3 4 5 6 7 8 9 10 Li Be Symbol Br B C N O F Ne 6.94 9.01 79.90 Atomic Weight 10.81 12.01 14.01 16.00 19.00 20.18 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar 8 9 10 22.99 24.31 3/IIIB 4/IVB 5/VB 6/VIB 7/VIIB VIIIBVIII 11/IB 12/IIB 26.98 28.09 30.97 32.07 35.45 39.95 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.87 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.41 69.72 72.64 74.92 78.9678.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (97.91)(98) 101.07 102.91 106.42 107.87 112.41 114.82 118.71 121.76 127.60 126.90 131.29 55 56 57–71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba La-Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn ´ 132.91 137.33 178.49 180.95 183.84 186.21 190.23 192.22 195.08 196.97 200.59 204.38 207.20207.2 208.98 (208.98)(209) (209.99)(210) (222.02)(222) 87 88 89–103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra AcAc-Lr - Lr Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo ´´ (223.02)(223) (226.03)(226) (261.11)(261) (262.11)(262) (266.12)(266) (264.12)(264) (277.00)(277) (268.14)(268) (247.07)(269) (280.00)(272) (285.00)(285) (284.00)(284) (289.00)(289) (288.00)(288) (293.00)(289) (294.00) (294.00)(294) ´ 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 138.91 140.12 140.91 144.24 (144.91)(145) 150.36 151.97151.96 157.25 158.93 162.50 164.93 167.26 168.93 173.04 174.97 US: www.wardsci.com Canada: www.wardsci.ca ´´ 800-962-2660 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 © 2010 Rev.