Investigation of Nitrogen Cycling Using Stable Nitrogen and Oxygen Isotopes in Narragansett Bay, Ri
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitrogen Isotope Variations in the Solar System Evelyn Füri and Bernard Marty
REVIEW ARTICLE PUBLISHED ONLINE: 15 JUNE 2015 | DOI: 10.1038/NGEO2451 Nitrogen isotope variations in the Solar System Evelyn Füri and Bernard Marty The relative proportion of the two isotopes of nitrogen, 14N and 15N, varies dramatically across the Solar System, despite little variation on Earth. NASA’s Genesis mission directly sampled the solar wind and confirmed that the Sun — and, by inference, the protosolar nebula from which the Solar System formed — is highly depleted in the heavier isotope compared with the reference nitrogen isotopic composition, that of Earth’s atmosphere. In contrast, the inner planets, asteroids, and comets are enriched in 15N by tens to hundreds of per cent; organic matter in primitive meteorites records the highest 15N/14N isotopic ratios. The measurements indicate that the protosolar nebula, inner Solar System, and cometary ices represent three distinct isotopic reservoirs, and that the 15N enrichment generally increases with distance from the Sun. The 15N enrichments were probably not inherited from presolar material, but instead resulted from nitrogen isotope fractionation processes that occurred early in Solar System history. Improvements in analytical techniques and spacecraft observations have made it possible to measure nitrogen isotopic variability in the Solar System at a level of accuracy that offers a window into the processing of early Solar System material, large-scale disk dynamics and planetary formation processes. he Solar System formed when a fraction of a dense molecu- a matter of debate, the D/H isotopic tracer offers the possibility of lar cloud collapsed and a central star, the proto-Sun, started investigating the relationships between the different Solar System Tburning its nuclear fuel1. -

Element Builder
Name: ______________________________________ Date: ________________________ Student Exploration: Element Builder Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. What are some of the different substances that make up a pizza? _____________________ _________________________________________________________________________ 2. What substances make up water? _____________________________________________ 3. What substances make up an iron pot? _________________________________________ Elements are pure substances that are made up of one kind of atom. Pizza is not an element because it is a mixture of many substances. Water is a pure substance, but it contains two kinds of atom: oxygen and hydrogen. Iron is an element because it is composed of one kind of atom. Gizmo Warm-up Atoms are tiny particles of matter that are made up of three particles: protons, neutrons, and electrons. The Element Builder Gizmo™ shows an atom with a single proton. The proton is located in the center of the atom, called the nucleus. 1. Use the arrow buttons ( ) to add protons, neutrons, and electrons to the atom. Press Play ( ). A. Which particles are located in the nucleus? _________________________________ B. Which particles orbit around the nucleus? __________________________________ 2. Turn on Show element name. What is the element? ________________________ 3. Manipulate the gizmo to figure out what actions cause the element name to change. _____________________________________________________________________ _____________________________________________________________________ Get the Gizmo ready: Activity A: Use the arrows to create an atom with two protons, Subatomic two neutrons, and two electrons. particles Turn on Show element name. Question: What are the properties of protons, neutrons, and electrons? 1. Observe: Turn on Show element symbol and Element notation. Three numbers surround the element symbol: the mass number, electrical charge (no number is displayed if the atom is neutral), and the atomic number. -

The Dole Effect and Its Variations During the Last 130,000 Years As Measured in the Vostok Ice Core
GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 8, NO. 3, PAGES 363-376, SEPTEMBER 1994 The Dole effect and its variations during the last 130,000 years as measured in the Vostok ice core Michael Bender • and Todd Sowers 2 GraduateSchool of Oceanography,University of RhodeIsland, Kingston Laurent Labeyrie Centredes Faibles Radioactivites, Centre National de la RechercheScientifique, Commissariat a L'EnergieAtomique, Gif-sur- Yvette, France Abstract. We review the currentunderstanding of the Dole effect (the observeddifference between the •5180of atmospheric0 2 andthat of seawater)and its causes, extend the record of variationsin theDole effectback to 130kyr before present using data on the •5180 of 0 2obtained from studying the Vostok ice core(Sowers et al., 1993), anddiscuss the significanceof temporalvariations. The Dole effectreflects oxygenisotope fractionation during photosynthesis, respiration, and hydrologic processes (evaporation, precipitation,and evapotranspiration). Our bestprediction of the present-dayDole effect,+20.8 %0,is considerablylower thanthe observedvalue, + 23.5 %0,and we discusspossible causes of this discrepancy. During the past 130 kyr, the Dole effecthas been 0.05 %0lower thanthe present value, on average.The standarddeviation of the Dole effect from the meanhas been only _+0.2 %0,and the Dole effect is nearly unchangedbetween glacial maxima and interglacial periods. The smallvariability in theDole effect suggeststhat relative rates of primaryproduction in theland and marine realms have been relatively constant.Most periodicvariability in the Dole effectis in the precessionband, suggesting that changes in thisglobal biogeochemical term reflects variations in low-latitudeland hydrology and productivity or possiblyvariability in low-latitudeoceanic productivity. Introduction they can potentially be made to do so. Ice core reconstructionsof the concentrationsof bioactivegases in air Our understanding of the response of the biosphere to provide an integratedglobal signal which complementsthe Pleistoceneclimate change is actually quite limited. -

Groundwater Quality Data for the Northern Sacramento Valley, 2007: Results from the California GAMA Program
Prepared in cooperation with the California State Water Resources Control Board A product of the California Groundwater Ambient Monitoring and Assessment (GAMA) Program Groundwater Quality Data for the Northern Sacramento Valley, 2007: Results from the California GAMA Program SHASTA CO Redding 5 Red Bluff TEHAMA CO Data Series 452 U.S. Department of the Interior U.S. Geological Survey Cover Photographs: Top: View looking down the fence line, 2008. (Photograph taken by Michael Wright, U.S. Geological Survey.) Bottom: A well/pump in a field, 2008. (Photograph taken by George Bennett, U.S. Geological Survey.) Groundwater Quality Data for the Northern Sacramento Valley, 2007: Results from the California GAMA Program By Peter A. Bennett, George L. Bennett V, and Kenneth Belitz In cooperation with the California State Water Resources Control Board Data Series 452 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Suzette M. Kimball, Acting Director U.S. Geological Survey, Reston, Virginia: 2009 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment, visit http://www.usgs.gov or call 1-888-ASK-USGS For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. -

12 Natural Isotopes of Elements Other Than H, C, O
12 NATURAL ISOTOPES OF ELEMENTS OTHER THAN H, C, O In this chapter we are dealing with the less common applications of natural isotopes. Our discussions will be restricted to their origin and isotopic abundances and the main characteristics. Only brief indications are given about possible applications. More details are presented in the other volumes of this series. A few isotopes are mentioned only briefly, as they are of little relevance to water studies. Based on their half-life, the isotopes concerned can be subdivided: 1) stable isotopes of some elements (He, Li, B, N, S, Cl), of which the abundance variations point to certain geochemical and hydrogeological processes, and which can be applied as tracers in the hydrological systems, 2) radioactive isotopes with half-lives exceeding the age of the universe (232Th, 235U, 238U), 3) radioactive isotopes with shorter half-lives, mainly daughter nuclides of the previous catagory of isotopes, 4) radioactive isotopes with shorter half-lives that are of cosmogenic origin, i.e. that are being produced in the atmosphere by interactions of cosmic radiation particles with atmospheric molecules (7Be, 10Be, 26Al, 32Si, 36Cl, 36Ar, 39Ar, 81Kr, 85Kr, 129I) (Lal and Peters, 1967). The isotopes can also be distinguished by their chemical characteristics: 1) the isotopes of noble gases (He, Ar, Kr) play an important role, because of their solubility in water and because of their chemically inert and thus conservative character. Table 12.1 gives the solubility values in water (data from Benson and Krause, 1976); the table also contains the atmospheric concentrations (Andrews, 1992: error in his Eq.4, where Ti/(T1) should read (Ti/T)1); 2) another category consists of the isotopes of elements that are only slightly soluble and have very low concentrations in water under moderate conditions (Be, Al). -

Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T
U.S. Department of the Interior U.S. Geological Survey Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T. B. Coplen', J. A. Hopple', J. K. Bohlke', H. S. Reiser', S. E. Rieder', H. R. o *< A R 1 fi Krouse , K. J. R. Rosman , T. Ding , R. D. Vocke, Jr., K. M. Revesz, A. Lamberty, P. Taylor, and P. De Bievre6 'U.S. Geological Survey, 431 National Center, Reston, Virginia 20192, USA 2The University of Calgary, Calgary, Alberta T2N 1N4, Canada 3Curtin University of Technology, Perth, Western Australia, 6001, Australia Institute of Mineral Deposits, Chinese Academy of Geological Sciences, Beijing, 100037, China National Institute of Standards and Technology, 100 Bureau Drive, Stop 8391, Gaithersburg, Maryland 20899 "institute for Reference Materials and Measurements, Commission of the European Communities Joint Research Centre, B-2440 Geel, Belgium Water-Resources Investigations Report 01-4222 Reston, Virginia 2002 U.S. Department of the Interior GALE A. NORTON, Secretary U.S. Geological Survey Charles G. Groat, Director The use of trade, brand, or product names in this report is for identification purposes only and does not imply endorsement by the U.S. Government. For additional information contact: Chief, Isotope Fractionation Project U.S. Geological Survey Mail Stop 431 - National Center Reston, Virginia 20192 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286 Denver, CO 80225-0286 CONTENTS Abstract...................................................... -
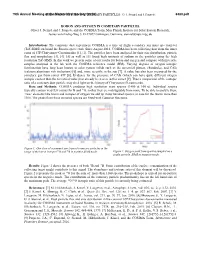
Boron and Oxygen in Cometary Particles: O. J
79th Annual Meeting ofBORON the Meteoritical AND OXYGEN Society IN COMETARY (2016) PARTICLES: O. J. Stenzel and J. Paquette 6380.pdf BORON AND OXYGEN IN COMETARY PARTICLES. Oliver J. Stenzel and J. Paquette and the COSIMA Team, Max Planck Institute for Solar System Research, Justus-von-Liebig-Weg 3, D-37077 Göttingen, Germany, [email protected]. Introduction: The cometary dust experiment COSIMA is a time of flight secondary ion mass spectrometer (ToF-SIMS) on board the Rosetta space craft. Since August 2014, COSIMA has been collecting dust from the inner coma of 67P/Churyumov-Gerasimenko [1], [2]. The particles have been analyzed for their size distribution, particle flux and morphology [3], [4]. [5] as well as [1] found high amounts of sodium in the particles using the high resolution ToF-SIMS. In this work we present some of our results for boron and oxygen and compare with meteorite samples analyzed in the lab with the COSIMA reference model (RM). Varying degrees of oxygen isotopic fractionation have long been known in solar system solids such as the terrestrial planets, chondrules, and CAIs (calcium-aluminum-rich inclusions) [6] and, more recently, in the sun [7]. A value has also been measured for the cometary gas from comet 67P [8]. Evidence for the presence of CAIs (which can have quite different oxygen isotopic content than the terrestrial value) has already been seen in this comet [9]. Thus a comparison of the isotopic ratio of a cometary dust particle may shed light on the history of Churyumov-Gerasimenko. Data and Methods: COSIMA produces high resolution mass spectra (1400 at 100 u). -

Analytical Developments in the Measurements of Boron, Nitrate
XA9848324 ANALYTICAL DEVELOPMENTS IN THE MEASUREMENTS OF BORON, NITRATE, PHOSPHATE AND SULPHATE ISOTOPES AND CASE EXAMPLES OF DISCRIMINATION OF NITROGEN AND SULPHUR SOURCES IN POLLUTION STUDIES J. AGGARWAL, D.S. SHEPPARD Institute of Geological & Nuclear Sciences, Lower Hutt B.W. ROBINSON Wairakei Research Centre, Institute of Geological & Nuclear Sciences, Taupo New Zealand Abstract - Methods are documented for the analysis of B isotopes, O and N isotopes in nitrates. B isotopes can be measured by negative ion thermal ionisation mass spectrometry. Nitrate is recovered from groundwaters by ion exchange and the resulting silver nitrate combusted for stable isotope gas analysis. Oxygen isotope analysis of phosphates can be determined by generating and analysing CO2 gas from the combustion of silver phosphate produced from aqueous samples. Sulphate in ground and surface waters can be separated and concentrated by ion exchange and precipitated as barium sulphate. This is reacted with graphite to yield CO2 and CO, the latter being spark discharged to CO2 and the total CO2 measured for oxygen isotope analysis. Barium sulphide from this reaction is converted to silver sulphide which is reacted with cuprous oxide to give SO2 gas for sulphur isotope measurements. A case study of the semi-rural Manakau area in New Zealand was conducted to see if nitrate isotopes could be used to detect the source of nitrate contamination (groundwater nitrate <0.5 to 11.3 mg/L NO'3-N). Nitrogen isotope (+4 to +12%o) coupled with oxygen isotope measurements (+5 to +9%o) demonstrated that the nitrogen is not sourced from fertilisers but from some combination of septic tank and animal waste. -

Using Stable Isotopes of Nitrogen and Carbon to Study Seabird Ecology: Applications in the Mediterranean Seabird Community*
SCI. MAR., 67 (Suppl. 2): 23-32 SCIENTIA MARINA 2003 MEDITERRANEAN SEABIRDS AND THEIR CONSERVATION. E. MÍNGUEZ, D. ORO, E. DE JUANA and A. MARTÍNEZ-ABRAÍN (eds.) Using stable isotopes of nitrogen and carbon to study seabird ecology: applications in the Mediterranean seabird community* MANUELA G. FORERO1 and KEITH A. HOBSON2 1 Instituto Mediterráneo de Estudios Avanzados (CSIC-UIB), C/Miquel Marqués, 21, 07190 Esporles, Mallorca, Spain. E-mail: [email protected] 2 Canadian Wildlife Service, 115 Perimeter Road, Saskatoon, SK, Canada, S7N0X4 SUMMARY: The application of the stable isotope technique to ecological studies is becoming increasingly widespread. In the case of seabirds, stable isotopes of nitrogen and carbon have been mainly used as dietary tracers. This approach relies on the fact that foodweb isotopic signatures are reflected in the tissues of the consumer. In addition to the study of trophic ecology, stable isotopes have been used to track the movement of seabirds across isotopic gradients, as individuals moving between isotopically distinct foodwebs can carry with them information on the location of previous feeding areas. Studies applying the stable isotope methodology to the study of seabird ecology show a clear evolution from broad and descriptive approaches to detailed and individual-based analyses. The purpose of this article is to show the different fields of applica- tion of stable isotopes to the study of the seabird ecology. Finally, we illustrate the utility of this technique by considering the particularities of the Mediterranean seabird community, suggesting different ecological questions and conservation prob- lems that could be addressed by using the stable isotope approach in this community. -

Reviewing Martian Atmospheric Noble Gas Measurements: from Martian Meteorites to Mars Missions
geosciences Review Reviewing Martian Atmospheric Noble Gas Measurements: From Martian Meteorites to Mars Missions Thomas Smith 1,* , P. M. Ranjith 1, Huaiyu He 1,2,3,* and Rixiang Zhu 1,2,3 1 State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, 19 Beitucheng Western Road, Box 9825, Beijing 100029, China; [email protected] (P.M.R.); [email protected] (R.Z.) 2 Institutions of Earth Science, Chinese Academy of Sciences, Beijing 100029, China 3 College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing 100049, China * Correspondence: [email protected] (T.S.); [email protected] (H.H.) Received: 10 September 2020; Accepted: 4 November 2020; Published: 6 November 2020 Abstract: Martian meteorites are the only samples from Mars available for extensive studies in laboratories on Earth. Among the various unresolved science questions, the question of the Martian atmospheric composition, distribution, and evolution over geological time still is of high concern for the scientific community. Recent successful space missions to Mars have particularly strengthened our understanding of the loss of the primary Martian atmosphere. Noble gases are commonly used in geochemistry and cosmochemistry as tools to better unravel the properties or exchange mechanisms associated with different isotopic reservoirs in the Earth or in different planetary bodies. The relatively low abundance and chemical inertness of noble gases enable their distributions and, consequently, transfer mechanisms to be determined. In this review, we first summarize the various in situ and laboratory techniques on Mars and in Martian meteorites, respectively, for measuring noble gas abundances and isotopic ratios. -

Stable Isotopes of Carbon and Nitrogen in the Study of Avian and Mammalian Trophic Ecology
Color profile: Disabled Composite Default screen 1 REVIEW/SYNTHÈSE Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology Jeffrey F. Kelly Abstract: Differential fractionation of stable isotopes of carbon during photosynthesis causes C4 plants and C3 plants to have distinct carbon-isotope signatures. In addition, marine C3 plants have stable-isotope ratios of carbon that are 13 12 intermediate between C4 and terrestrial C3 plants. The direct incorporation of the carbon-isotope ratio ( C/ C) of plants into consumers’ tissues makes this ratio useful in studies of animal ecology. The heavy isotope of nitrogen (15N) is preferentially incorporated into the tissues of the consumer from the diet, which results in a systematic enrichment in nitrogen-isotope ratio (15N/14N) with each trophic level. Consequently, stable isotopes of nitrogen have been used pri- marily to assess position in food chains. The literature pertaining to the use of stable isotopes of carbon and nitrogen in animal trophic ecology was reviewed. Data from 102 studies that reported stable-isotope ratios of carbon and (or) nitrogen of wild birds and (or) mammals were compiled and analyzed relative to diet, latitude, body size, and habitat moisture. These analyses supported the predicted relationships among trophic groups. Carbon-isotope ratios differed among species that relied on C3,C4, and marine food chains. Likewise, nitrogen-isotope ratios were enriched in terres- trial carnivorous mammals relative to terrestrial herbivorous mammals. -

Isotopes of Nitrogen on Mars: Atmospheric Measurements by Curiosity’S Mass Spectrometer Michael H
GEOPHYSICAL RESEARCH LETTERS, VOL. ???, XXXX, DOI:10.1029/, Isotopes of nitrogen on Mars: Atmospheric measurements by Curiosity's mass spectrometer Michael H. Wong,1,2 Sushil K. Atreya,1 Paul N. Mahaffy,3 Heather B. Franz,3 Charles Malespin,3 Melissa M. Trainer,3 Jennifer C. Stern,3 Pamela G. Conrad,3 Heidi L.K. Manning,4 Robert O. Pepin,5 Richard H. Becker,5 Christopher P. McKay,6 Tobias C. Owen,7 Rafael Navarro-Gonz´alez,8 John H. Jones,9 Bruce M. Jakosky,10 and Andrew Steele11 The Sample Analysis at Mars (SAM) instrument suite on atmosphere and surface of Mars is not currently understood, the Mars Science Laboratory (MSL) measured a Mars at- but such exchange would be very important to the study of mospheric 14N/15N ratio of 173 ± 11 on sol 341 of the mis- martian habitability that is the focus of the MSL mission [Grotzinger et al., 2012]. sion, agreeing with Viking's measurement of 168 ± 17 [Nier The atmospheric nitrogen isotopic ratio is difficult to and McElroy, 1977]. The MSL/SAM value was based on measure. Very weak absorption lines are produced by the Quadrupole Mass Spectrometer (QMS) measurements of an dipole forbidden vibrational-rotational transitions of ground enriched atmospheric sample, with CO2 and H2O removed. state N2, so the spectroscopic techniques used to measure Doubly-ionized nitrogen data at m/z 14 and 14.5 had the isotope ratios in other molecules cannot be applied to molec- highest signal/background ratio, with results confirmed by ular nitrogen. Of the 10 science instruments of the MSL m/z 28 and 29 data.