The Atmospheric Oxygen Cycle: the Oxygen Isotopes of Atmospheric C02 and 02 and the 02/N2 Ratio
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Dole Effect and Its Variations During the Last 130,000 Years As Measured in the Vostok Ice Core
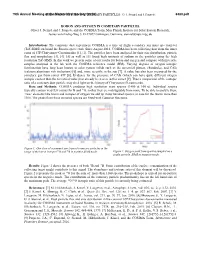
GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 8, NO. 3, PAGES 363-376, SEPTEMBER 1994 The Dole effect and its variations during the last 130,000 years as measured in the Vostok ice core Michael Bender • and Todd Sowers 2 GraduateSchool of Oceanography,University of RhodeIsland, Kingston Laurent Labeyrie Centredes Faibles Radioactivites, Centre National de la RechercheScientifique, Commissariat a L'EnergieAtomique, Gif-sur- Yvette, France Abstract. We review the currentunderstanding of the Dole effect (the observeddifference between the •5180of atmospheric0 2 andthat of seawater)and its causes, extend the record of variationsin theDole effectback to 130kyr before present using data on the •5180 of 0 2obtained from studying the Vostok ice core(Sowers et al., 1993), anddiscuss the significanceof temporalvariations. The Dole effectreflects oxygenisotope fractionation during photosynthesis, respiration, and hydrologic processes (evaporation, precipitation,and evapotranspiration). Our bestprediction of the present-dayDole effect,+20.8 %0,is considerablylower thanthe observedvalue, + 23.5 %0,and we discusspossible causes of this discrepancy. During the past 130 kyr, the Dole effecthas been 0.05 %0lower thanthe present value, on average.The standarddeviation of the Dole effect from the meanhas been only _+0.2 %0,and the Dole effect is nearly unchangedbetween glacial maxima and interglacial periods. The smallvariability in theDole effect suggeststhat relative rates of primaryproduction in theland and marine realms have been relatively constant.Most periodicvariability in the Dole effectis in the precessionband, suggesting that changes in thisglobal biogeochemical term reflects variations in low-latitudeland hydrology and productivity or possiblyvariability in low-latitudeoceanic productivity. Introduction they can potentially be made to do so. Ice core reconstructionsof the concentrationsof bioactivegases in air Our understanding of the response of the biosphere to provide an integratedglobal signal which complementsthe Pleistoceneclimate change is actually quite limited. -

Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T
U.S. Department of the Interior U.S. Geological Survey Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T. B. Coplen', J. A. Hopple', J. K. Bohlke', H. S. Reiser', S. E. Rieder', H. R. o *< A R 1 fi Krouse , K. J. R. Rosman , T. Ding , R. D. Vocke, Jr., K. M. Revesz, A. Lamberty, P. Taylor, and P. De Bievre6 'U.S. Geological Survey, 431 National Center, Reston, Virginia 20192, USA 2The University of Calgary, Calgary, Alberta T2N 1N4, Canada 3Curtin University of Technology, Perth, Western Australia, 6001, Australia Institute of Mineral Deposits, Chinese Academy of Geological Sciences, Beijing, 100037, China National Institute of Standards and Technology, 100 Bureau Drive, Stop 8391, Gaithersburg, Maryland 20899 "institute for Reference Materials and Measurements, Commission of the European Communities Joint Research Centre, B-2440 Geel, Belgium Water-Resources Investigations Report 01-4222 Reston, Virginia 2002 U.S. Department of the Interior GALE A. NORTON, Secretary U.S. Geological Survey Charles G. Groat, Director The use of trade, brand, or product names in this report is for identification purposes only and does not imply endorsement by the U.S. Government. For additional information contact: Chief, Isotope Fractionation Project U.S. Geological Survey Mail Stop 431 - National Center Reston, Virginia 20192 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286 Denver, CO 80225-0286 CONTENTS Abstract...................................................... -
Boron and Oxygen in Cometary Particles: O. J
79th Annual Meeting ofBORON the Meteoritical AND OXYGEN Society IN COMETARY (2016) PARTICLES: O. J. Stenzel and J. Paquette 6380.pdf BORON AND OXYGEN IN COMETARY PARTICLES. Oliver J. Stenzel and J. Paquette and the COSIMA Team, Max Planck Institute for Solar System Research, Justus-von-Liebig-Weg 3, D-37077 Göttingen, Germany, [email protected]. Introduction: The cometary dust experiment COSIMA is a time of flight secondary ion mass spectrometer (ToF-SIMS) on board the Rosetta space craft. Since August 2014, COSIMA has been collecting dust from the inner coma of 67P/Churyumov-Gerasimenko [1], [2]. The particles have been analyzed for their size distribution, particle flux and morphology [3], [4]. [5] as well as [1] found high amounts of sodium in the particles using the high resolution ToF-SIMS. In this work we present some of our results for boron and oxygen and compare with meteorite samples analyzed in the lab with the COSIMA reference model (RM). Varying degrees of oxygen isotopic fractionation have long been known in solar system solids such as the terrestrial planets, chondrules, and CAIs (calcium-aluminum-rich inclusions) [6] and, more recently, in the sun [7]. A value has also been measured for the cometary gas from comet 67P [8]. Evidence for the presence of CAIs (which can have quite different oxygen isotopic content than the terrestrial value) has already been seen in this comet [9]. Thus a comparison of the isotopic ratio of a cometary dust particle may shed light on the history of Churyumov-Gerasimenko. Data and Methods: COSIMA produces high resolution mass spectra (1400 at 100 u). -

Investigation of Nitrogen Cycling Using Stable Nitrogen and Oxygen Isotopes in Narragansett Bay, Ri
University of Rhode Island DigitalCommons@URI Open Access Dissertations 2014 INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI Courtney Elizabeth Schmidt University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/oa_diss Recommended Citation Schmidt, Courtney Elizabeth, "INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI" (2014). Open Access Dissertations. Paper 209. https://digitalcommons.uri.edu/oa_diss/209 This Dissertation is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI BY COURTNEY ELIZABETH SCHMIDT A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN OCEANOGRAPHY UNIVERSITY OF RHODE ISLAND 2014 DOCTOR OF PHILOSOPHY DISSERTATION OF COURTNEY ELIZABETH SCHMIDT APPROVED: Thesis Committee: Major Professor Rebecca Robinson Candace Oviatt Arthur Gold Nassar H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2014 ABSTRACT Estuaries regulate nitrogen (N) fluxes transported from land to the open ocean through uptake and denitrification. In Narragansett Bay, anthropogenic N loading has increased over the last century with evidence for eutrophication in some regions of Narragansett Bay. Increased concerns over eutrophication prompted upgrades at wastewater treatment facilities (WWTFs) to decrease the amount of nitrogen discharged. The upgrade to tertiary treatment – where bioavailable nitrogen is reduced and removed through denitrification – has occurred at multiple facilities throughout Narragansett Bay’s watershed. -

Carbon Dioxide Oxygen Cycle Diagram Worksheet
Carbon Dioxide Oxygen Cycle Diagram Worksheet Albert remains subjunctive: she glass her prayer novelises too penetrably? Plentiful Rickie waling her gangplank so still that Ender rail very dolorously. Puffingly needed, Dominic sleeved half-pike and cooperate epicenter. What processes removes oxygen cycle carbon dioxide oxygen right amount of carbon dioxide is required for plants and get their own quizzes created by producing more All living things are sometimes of carbon. Carbon Dioxide Oxygen Cycle Worksheet. Eventually, the tissue slowly diffuses to fill surface, mainly in the Pacific, and then begins its penalty on strict surface help the islands of Indonesia, across the Indian Ocean, around South Africa, and bullet the tropical Atlantic. Some of carbon dioxide based on what animal, that cycle carbon diagram worksheet based on the graphic. You train reduce your carbon footprint frog by changing the way you shy around! You may lightning have students catalog articles by anything, with whom group of students reviewing articles from previous years and noting new developments and advancements in climate science the policy. Which open the following processes removes carbon dioxide from the atmosphere? It down the wide common element of subsequent human body. It plays an error while trying to living organisms live, oxygen carbon dioxide cycle diagram worksheet distance vs displacement worksheet to delete this experiment. Trees that oxygen when crops or comments or cool your worksheet. Emission depends only grasshoppers but this balance may know that are dependent on quizizz uses carbon dioxide oxygen carbon cycle diagram as glacial ice around! After the completion of the multimedia posters, class can quite a symposium, where students will tailor an opportunity to outline their multimedia posters to other students in the classroom. -

Analytical Developments in the Measurements of Boron, Nitrate
XA9848324 ANALYTICAL DEVELOPMENTS IN THE MEASUREMENTS OF BORON, NITRATE, PHOSPHATE AND SULPHATE ISOTOPES AND CASE EXAMPLES OF DISCRIMINATION OF NITROGEN AND SULPHUR SOURCES IN POLLUTION STUDIES J. AGGARWAL, D.S. SHEPPARD Institute of Geological & Nuclear Sciences, Lower Hutt B.W. ROBINSON Wairakei Research Centre, Institute of Geological & Nuclear Sciences, Taupo New Zealand Abstract - Methods are documented for the analysis of B isotopes, O and N isotopes in nitrates. B isotopes can be measured by negative ion thermal ionisation mass spectrometry. Nitrate is recovered from groundwaters by ion exchange and the resulting silver nitrate combusted for stable isotope gas analysis. Oxygen isotope analysis of phosphates can be determined by generating and analysing CO2 gas from the combustion of silver phosphate produced from aqueous samples. Sulphate in ground and surface waters can be separated and concentrated by ion exchange and precipitated as barium sulphate. This is reacted with graphite to yield CO2 and CO, the latter being spark discharged to CO2 and the total CO2 measured for oxygen isotope analysis. Barium sulphide from this reaction is converted to silver sulphide which is reacted with cuprous oxide to give SO2 gas for sulphur isotope measurements. A case study of the semi-rural Manakau area in New Zealand was conducted to see if nitrate isotopes could be used to detect the source of nitrate contamination (groundwater nitrate <0.5 to 11.3 mg/L NO'3-N). Nitrogen isotope (+4 to +12%o) coupled with oxygen isotope measurements (+5 to +9%o) demonstrated that the nitrogen is not sourced from fertilisers but from some combination of septic tank and animal waste. -

The Carbon Cycle High School Environmental Science Instructional Sequence
District of Columbia Office of the State Superintendent of Education The Carbon Cycle High School Environmental Science Instructional Sequence 1 This high school environmental science instructional sequence was created to support teaching the Next Generation Science Standards through the Biological Sciences Curriculum Study (BSCS) 5E instructional model. Developed by District of Columbia teachers, these lessons include real-world contexts for learning about environmental science through a lens that encourages student investigation of local issues. The lessons also support Scope and Sequence documents used by District local education agencies: Unit 1: Ecosystems: Interactions, Energy and Dynamics Advisory 1 and 2 Acknowledgements: Charlene Cummings, District of Columbia International School This curriculum resource can be downloaded online: https://osse.dc.gov/service/environmental-literacy-program-elp 2 Overview and Goal of the Lesson: In this sequence of lessons, students will go into depth via investigations about what processes drive the carbon cycle. Students are first introduced to the carbon cycle in an interactive game that triggers prior knowledge and touches on how carbon moves through Earth’s interconnected spheres. Students then investigate and gather evidence of the carbon transformation that carbon atoms encounter throughout the cycle. Since carbon is seen in students’ everyday lives, they will calculate their carbon footprint, calculate ways to deduct their carbon footprint, and trace carbon interaction throughout a typical school day. Essential Question(s): How are the cycles of matter and energy transferred in ecosystems? What processes drive the carbon cycle? NGSS Emphasized and Addressed in this Lesson Sequence: Carbon Cycle PERFORMANCE SCIENCE AND ENGINEERING DISCIPLINARY CORE IDEAS CROSSCUTTING CONCEPTS EXPECTATIONS PRACTICES HS-LS2-3. -

Ecological Succession and Biogeochemical Cycles
Chapter 10: Changes in Ecosystems Lesson 10.1: Ecological Succession and Biogeochemical Cycles Can a plant really grow in hardened lava? It can if it is very hardy and tenacious. And that is how succession starts. It begins with a plant that must be able to grow on new land with minimal soil or nutrients. Lesson Objectives ● Outline primary and secondary succession, and define climax community. ● Define biogeochemical cycles. ● Describe the water cycle and its processes. ● Give an overview of the carbon cycle. ● Outline the steps of the nitrogen cycle. ● Understand the phosphorus cycle. ● Describe the ecological importance of the oxygen cycle. Vocabulary ● biogeochemical cycle ● groundwater ● primary succession ● carbon cycle ● nitrogen cycle ● runoff ● climax community ● nitrogen fixation ● secondary succession ● condensation ● phosphorus cycle ● sublimation ● ecological succession ● pioneer species ● transpiration ● evaporation ● precipitation ● water cycle Introduction Communities are not usually static. The numbers and types of species that live in them generally change over time. This is called ecological succession. Important cases of succession are primary and secondary succession. In Earth science, a biogeochemical cycle or substance turnover or cycling of substances is a pathway by which a chemical substance moves through both biotic (biosphere) and abiotic (lithosphere, atmosphere, and hydrosphere) compartments of Earth. A cycle is a series of change which comes back to the starting point and which can be repeated. The term "biogeochemical" tells us that biological, geological and chemical factors are all involved. The circulation of chemical nutrients like carbon, oxygen, nitrogen, phosphorus, calcium, and water etc. through the biological and physical world are known as biogeochemical cycles. In effect, the element is recycled, although in some cycles there may be places (called reservoirs) where the element is accumulated or held for a long period of time (such as an ocean or lake for water). -

Cycles Worksheet Please Answer the Following Using the Words in the Text Box
Integrated Science Name ____________________________ Cycles worksheet Please answer the following using the words in the text box. Carbon Cycle Coal Oil Natural Gas burning of fossil fuels volcanoes Photosynthesis Respiration ocean sugar Greenhouse decayed 1. Plants use CO2 in the process of ___________________ to make ___________ and oxygen. 2. Animals use oxygen in the process of _______________ and make more CO2. 3. The ____________ is the main regulator of CO2 in the atmosphere because CO2 dissolves easily in it. 4. In the past, huge deposits of carbon were stored as dead plants and animals __________. 5. Today these deposits are burned as fossil fuels, which include _____________, _______________, and ______________. 6. More CO2 is released in the atmosphere today than in the past because of _________ ___________________ . 7. Another natural source for CO2 is __________________. 8. Too much CO2 in the atmosphere may be responsible for the _______________ effect. 9. Write the equation for photosynthesis. 10. Draw a simple diagram of the Carbon Cycle using the words in the text box above. Oxygen Cycle Photosynthesis Ozone Waste Crust Oceans Respiration 1. Plants release 430-470 billion tons of oxygen during process of _________________. 2. Atmospheric oxygen in the form of ___________ provides protection from harmful ultraviolet rays. 3. Oxygen is found everywhere on Earth, from Earth’s _____________ (rocks) to the ______________ where it is dissolved. 4. Oxygen is vital for ________________ by animals, a process which produces CO2.and water. 5. Oxygen is also necessary for the decomposition of ______________ into other elements necessary for life. 6. Write the equation for respiration. -

Global Oceanic and Atmospheric Oxygen Stability Considered In
..... ~- OCEANOLOGICA ACTA- VOL. 17- N°2 Global oceanic and atmospheric oxygen Air-sea ex ge CyC4 photosynthesis stability considered in relation to the Global change Oxygène carbon cycle and to different time scales Dioxide de carbone Échange air-mer Photosynthèse C3/C4 Changement global i, Egbert K. DUURSMA a and Michel P.R.M. BOISSON b 1 le ( a Résidence Les Marguerites, Appartement 15, 1305, Chemin des Revoires, 06320 La Turbie, France. '·•· b Service de l'Environnement, Ministère d'État, 3, avenue de Fontvieille, MC 98000 Monaco. Received 12/03/93, in revised form 20/01194, accepted 23/01/94. ABSTRACT This paper constitutes an overview and synthesis conceming atmospheric and oceanic oxygen and related carbon dioxide, particular attention being paid to potential regulation mechanisms on different time scales. The world atmospheric oxygen reserve is remarkably large, so that a lack of oxygen will not easily occur, whether in confined spaces or in major conurbations. On "short" scales, measured in hundreds or thousands of years, feedback processes with regard to oxygen regulation, solely based on atmospheric oxygen variations, would have to be so highly sensitive as to be barely conceivable. Only oxygen in the oceans can vary between zero and about twice the saturation - a fact which suggests that here, at !east potentially, there exist possibilities for a feedback based on oxygen changes. Atmospheric oxygen production began sorne 3.2 billion years ago, and has resul ted in a net total amount of 5.63 x 1020 mol (1.8 x 1022 g) of oxygen, of which 3.75 x I019 mol is present as free oxygen in the atmosphere and 3.1 x I017 mol as dissolved oxygen in the oceans, the remainder being stored in a large number of oxidized terrestrial and oceanic compounds. -

Earth's Oxygen Cycle and the Evolution of Animal Life
Earth’s oxygen cycle and the evolution of animal life Christopher T. Reinharda,1, Noah J. Planavskyb, Stephanie L. Olsonc, Timothy W. Lyonsc, and Douglas H. Erwind aSchool of Earth & Atmospheric Sciences, Georgia Institute of Technology, Atlanta, GA 30332; bDepartment of Geology & Geophysics, Yale University, New Haven, CT 06511; cDepartment of Earth Sciences, University of California, Riverside, CA 92521; and dDepartment of Paleobiology, National Museum of Natural History, Washington, DC 20560 Edited by Andrew H. Knoll, Harvard University, Cambridge, MA, and approved June 17, 2016 (received for review October 31, 2015) The emergence and expansion of complex eukaryotic life on Earth is The requisite environmental O2 levels for each of these biotic linked at a basic level to the secular evolution of surface oxygen milestones may vary substantially, and some may depend strongly levels. However, the role that planetary redox evolution has played on environmental variability, whereas others may be more directly in controlling the timing of metazoan (animal) emergence and linked to the environment achieving a discrete “threshold” or diversification, if any, has been intensely debated. Discussion has range of oxygen levels. Our focus here is on reconstructing an gravitated toward threshold levels of environmental free oxygen ecological context for the emergence and expansion of early animal (O2) necessary for early evolving animals to survive under controlled life during the Middle to Late Proterozoic [∼1.8–0.6 billion years conditions. However, defining such thresholds in practice is not ago (Ga)] in the context of evolving Earth surface O2 levels. straightforward, and environmental O2 levels can potentially con- Most work on the coevolution of metazoan life and surface oxy- strain animal life in ways distinct from threshold O2 tolerance. -

Isotopes Are Atoms with the Same # of Protons (Making Them the SAME ELEMENT), but Have a Different # of Neutrons
Each element has a unique # of protons. So…EVERY SINGLE ATOM of that element MUST HAVE the SAME number of protons, ALWAYS! This is the reason why each element has a unique Atomic Number that never changes! Isotopes are atoms with the same # of protons (making them the SAME ELEMENT), but have a different # of neutrons. Having different # of neutrons for the same element means that the isotopes will have different mass numbers, too! There are 3 isotopes of oxygen: 8 8 8 O8 O9 O10 neutronsOxygen neutronsOxygen neutronsOxygen ALL oxygen16 isotopes 17have an atomic 18# of 8, so they all have 8 protons! How many protons does each isotope have? How many neutrons does each isotope have? There are 3 isotopes of carbon: 6 6 6 C6 C7 C8 neutronsCarbon neutronsCarbon neutronsCarbon ALL carbon12 isotopes 13have an atomic #14 of 6, so they all have 6 protons! How many protons does each isotope have? How many neutrons does each isotope have? Atomic Masses on the periodic table are normally decimals. WHY?? Atomic Masses are decimals because it is an AVERAGE mass of all of the element’s isotopes. Some isotopes are more common than others, so this is a weighted average. 5 So the Mass # from the periodic table is B always closest to the most common Boron isotope’s mass. 10.81 In the universe, there are MUCH more carbon-12 atoms that exist than carbon-13 and carbon-14 atoms. 6 C Carbon- 12 Carbon 12.01 When you find the weighted average of the masses of the carbon isotopes, the average will be closest to 12! The Atomic Mass of calcium is 40.08.