How Many Named Species Are Valid?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paleontological Resources of the Upper and Middle San Pedro Valley
Paleontological Resources of the Upper and Middle San Pedro Valley Robert D. McCord Arizona Museum of Natural History Geological setting Regional extension causing block faulting – creation of the Basin and Range ~15Ma Poorly developed drainage results in lakes in valley bottom ?-3.4 Ma Drainage develops with flow to north, marshes, ponds and lakes significant from time to time Early Pleistocene Saint David Formation ? – 3.4 million lakes, few fossils Well developed paleomagnetic timeframe – a first for terrestrial sediments! Succession of faunas from ~3 to 1.5 Ma Blancan to ? Irvingtonian NALMA Plants diatoms charophytes Equisetum (scouring rush) Ostracoda (aquatic crustaceans) Cypridopsis cf. vidua Limnocythere cf. staplini Limnocythere sp. Candona cf. renoensis Candona sp. A Candona sp. B ?Candonlella sp. ?Heterocypris sp. ?Cycloypris sp. Potamocypris sp. Cyprideis sp. Darwinula sp. Snails and a Clam Pisidium casertanum (clam) Fossaria dalli (aquatic snail) Lymnaea caperata (aquatic snail) Lymnaea cf. elodes (aquatic snail) Bakerilymnaea bulimoides (aquatic snail) Gyraulus parvus (aquatic snail) Promenetus exacuous (aquatic snail) Promenetus umbilicatellus (aquatic snail) Physa virgata (aquatic snail) Gastrocopta cristata (terrestrial snail) Gastrocopta tappaniana (terrestrial snail) Pupoides albilabris (terrestrial snail) Vertigo milium (terrestrial snail) Vertigo ovata (terrestrial snail) cf. Succinea (terrestrial snail) Deroceras aenigma (slug) Hawaila minuscula (terrestrial snail) Fish and Amphibians indeterminate small fish Ambystoma tigrinum (tiger salamander) Scaphiopus hammondi (spadefoot toad) Bufo alvarius (toad) Hyla eximia (tree frog) Rana sp. (leopard frog) Turtles and Lizards Kinosternon arizonense (mud turtle) Terrapene cf. ornata (box turtle) Gopherus sp. (tortoise) Hesperotestudo sp. (giant tortoise) Eumeces sp. (skink) “Cnemidophorus” sp. (whiptail lizard) Crotaphytus sp. (collared lizard) Phrynosoma sp. (horned lizard) Sceloporus sp. -

La Brea and Beyond: the Paleontology of Asphalt-Preserved Biotas
La Brea and Beyond: The Paleontology of Asphalt-Preserved Biotas Edited by John M. Harris Natural History Museum of Los Angeles County Science Series 42 September 15, 2015 Cover Illustration: Pit 91 in 1915 An asphaltic bone mass in Pit 91 was discovered and exposed by the Los Angeles County Museum of History, Science and Art in the summer of 1915. The Los Angeles County Museum of Natural History resumed excavation at this site in 1969. Retrieval of the “microfossils” from the asphaltic matrix has yielded a wealth of insect, mollusk, and plant remains, more than doubling the number of species recovered by earlier excavations. Today, the current excavation site is 900 square feet in extent, yielding fossils that range in age from about 15,000 to about 42,000 radiocarbon years. Natural History Museum of Los Angeles County Archives, RLB 347. LA BREA AND BEYOND: THE PALEONTOLOGY OF ASPHALT-PRESERVED BIOTAS Edited By John M. Harris NO. 42 SCIENCE SERIES NATURAL HISTORY MUSEUM OF LOS ANGELES COUNTY SCIENTIFIC PUBLICATIONS COMMITTEE Luis M. Chiappe, Vice President for Research and Collections John M. Harris, Committee Chairman Joel W. Martin Gregory Pauly Christine Thacker Xiaoming Wang K. Victoria Brown, Managing Editor Go Online to www.nhm.org/scholarlypublications for open access to volumes of Science Series and Contributions in Science. Natural History Museum of Los Angeles County Los Angeles, California 90007 ISSN 1-891276-27-1 Published on September 15, 2015 Printed at Allen Press, Inc., Lawrence, Kansas PREFACE Rancho La Brea was a Mexican land grant Basin during the Late Pleistocene—sagebrush located to the west of El Pueblo de Nuestra scrub dotted with groves of oak and juniper with Sen˜ora la Reina de los A´ ngeles del Rı´ode riparian woodland along the major stream courses Porciu´ncula, now better known as downtown and with chaparral vegetation on the surrounding Los Angeles. -
Download PDF File
1.08 1.19 1.46 Nimravus brachyops Nandinia binotata Neofelis nebulosa 115 Panthera onca 111 114 Panthera atrox 113 Uncia uncia 116 Panthera leo 112 Panthera pardus Panthera tigris Lynx issiodorensis 220 Lynx rufus 221 Lynx pardinus 222 223 Lynx canadensis Lynx lynx 119 Acinonyx jubatus 110 225 226 Puma concolor Puma yagouaroundi 224 Felis nigripes 228 Felis chaus 229 Felis margarita 118 330 227 331Felis catus Felis silvestris 332 Otocolobus manul Prionailurus bengalensis Felis rexroadensis 99 117 334 335 Leopardus pardalis 44 333 Leopardus wiedii 336 Leopardus geoffroyi Leopardus tigrinus 337 Pardofelis marmorata Pardofelis temminckii 440 Pseudaelurus intrepidus Pseudaelurus stouti 88 339 Nimravides pedionomus 442 443 Nimravides galiani 22 338 441 Nimravides thinobates Pseudaelurus marshi Pseudaelurus validus 446 Machairodus alberdiae 77 Machairodus coloradensis 445 Homotherium serum 447 444 448 Smilodon fatalis Smilodon gracilis 66 Pseudaelurus quadridentatus Barbourofelis morrisi 449 Barbourofelis whitfordi 550 551 Barbourofelis fricki Barbourofelis loveorum Stenogale Hemigalus derbyanus 554 555 Arctictis binturong 55 Paradoxurus hermaphroditus Genetta victoriae 553 558 Genetta maculata 559 557 660 Genetta genetta Genetta servalina Poiana richardsonii 556 Civettictis civetta 662 Viverra tangalunga 661 663 552 Viverra zibetha Viverricula indica Crocuta crocuta 666 667 Hyaena brunnea 665 Hyaena hyaena Proteles cristata Fossa fossana 664 669 770 Cryptoprocta ferox Salanoia concolor 668 772 Crossarchus alexandri 33 Suricata suricatta 775 -

PHYLOGENETIC SYSTEMATICS of the BOROPHAGINAE (CARNIVORA: CANIDAE) Xiaoming Wang
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Mammalogy Papers: University of Nebraska State Museum, University of Nebraska State Museum 1999 PHYLOGENETIC SYSTEMATICS OF THE BOROPHAGINAE (CARNIVORA: CANIDAE) Xiaoming Wang Richard H. Tedford Beryl E. Taylor Follow this and additional works at: http://digitalcommons.unl.edu/museummammalogy This Article is brought to you for free and open access by the Museum, University of Nebraska State at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Mammalogy Papers: University of Nebraska State Museum by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. PHYLOGENETIC SYSTEMATICS OF THE BOROPHAGINAE (CARNIVORA: CANIDAE) XIAOMING WANG Research Associate, Division of Paleontology American Museum of Natural History and Department of Biology, Long Island University, C. W. Post Campus, 720 Northern Blvd., Brookville, New York 11548 -1300 RICHARD H. TEDFORD Curator, Division of Paleontology American Museum of Natural History BERYL E. TAYLOR Curator Emeritus, Division of Paleontology American Museum of Natural History BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 243, 391 pages, 147 figures, 2 tables, 3 appendices Issued November 17, 1999 Price: $32.00 a copy Copyright O American Museum of Natural History 1999 ISSN 0003-0090 AMNH BULLETIN Monday Oct 04 02:19 PM 1999 amnb 99111 Mp 2 Allen Press • DTPro System File # 01acc (large individual, composite figure, based Epicyon haydeni ´n. (small individual, based on AMNH 8305) and Epicyon saevus Reconstruction of on specimens from JackNorth Swayze America. Quarry). Illustration These by two Mauricio species Anto co-occur extensively during the late Clarendonian and early Hemphillian of western 2 1999 WANG ET AL.: SYSTEMATICS OF BOROPHAGINAE 3 CONTENTS Abstract .................................................................... -
First Bone-Cracking Dog Coprolites Provide New Insight
RESEARCH ARTICLE First bone-cracking dog coprolites provide new insight into bone consumption in Borophagus and their unique ecological niche Xiaoming Wang1,2,3*, Stuart C White4, Mairin Balisi1,3, Jacob Biewer5,6, Julia Sankey6, Dennis Garber1, Z Jack Tseng1,2,7 1Department of Vertebrate Paleontology, Natural History Museum of Los Angeles County, Los Angeles, United States; 2Department of Vertebrate Paleontology, American Museum of Natural History, New York, United States; 3Department of Ecology and Evolutionary Biology, University of California, Los Angeles, United States; 4School of Dentistry, University of California, Los Angeles, United States; 5Department of Geological Sciences, California State University, Fullerton, United States; 6Department of Geology, California State University Stanislaus, Turlock, United States; 7Department of Pathology and Anatomical Sciences, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, United States Abstract Borophagine canids have long been hypothesized to be North American ecological ‘avatars’ of living hyenas in Africa and Asia, but direct fossil evidence of hyena-like bone consumption is hitherto unknown. We report rare coprolites (fossilized feces) of Borophagus parvus from the late Miocene of California and, for the first time, describe unambiguous evidence that these predatory canids ingested large amounts of bone. Surface morphology, micro-CT analyses, and contextual information reveal (1) droppings in concentrations signifying scent-marking behavior, similar to latrines used by living social carnivorans; (2) routine consumption of skeletons; *For correspondence: (3) undissolved bones inside coprolites indicating gastrointestinal similarity to modern striped and [email protected] brown hyenas; (4) B. parvus body weight of ~24 kg, reaching sizes of obligatory large-prey hunters; ~ Competing interests: The and (5) prey size ranging 35–100 kg. -

Leeds Thesis Template
Middle to Late Miocene terrestrial biota and climate by Matthew James Pound M.Sci., Geology (University of Bristol) Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University of Leeds School of Earth and Environment September 2012 - 2 - Declaration of Authorship The candidate confirms that the work submitted is his/her own, except where work which has formed part of jointly-authored publications has been included. The contribution of the candidate and the other authors to this work has been explicitly indicated below. The candidate confirms that appropriate credit has been given within the thesis where reference has been made to the work of others. Chapter 2 has been published as: Pound, M.J., Riding, J.B., Donders, T.H., Daskova, J. 2012 The palynostratigraphy of the Brassington Formation (Upper Miocene) of the southern Pennines, central England. Palynology 36, 26-37. Chapter 3 has been published as: Pound, M.J., Haywood, A.M., Salzmann, U., Riding, J.B. 2012. Global vegetation dynamics and latitudinal temperature gradients during the mid to Late Miocene (15.97 - 5.33 Ma). Earth Science Reviews 112, 1-22. Chapter 4 has been published as: Pound, M.J., Haywood, A.M., Salzmann, U., Riding, J.B., Lunt, D.J. and Hunter, S.J. 2011. A Tortonian (Late Miocene 11.61-7.25Ma) global vegetation reconstruction. Palaeogeography, Palaeoclimatology, Palaeoecology 300, 29-45. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. © 2012, The University of Leeds, British Geological Survey and Matthew J. -

1: Beast2.1.3 R1
1: Beast2.1.3_r1 0.93 Canis adustus 0.89 Canis mesomelas 0.37 Canis simensis Canis arnensis 1 Lycaon pictus 1 Cuon javanicus 0.57 Xenocyon texanus 0.91 Canis antonii 0.96 0.35 Canis falconeri 1 Canis dirus 1 Canis armbrusteri 0.060.27 Canis lupus Canis chihliensis Canis etruscus 0.98 0.99 0.26 Canis variabilis 0.11 Canis edwardii 0.93 Canis mosbachensis 0.68 0.58 Canis latrans Canis aureus 0.94 Canis palmidens 0.94 Canis lepophagus 1 0.41 Canis thooides Canis ferox 0.99 Eucyon davisi 0.8 Cerdocyon texanus Vulpes stenognathus Leptocyon matthewi 0.98 Urocyon cinereoargenteus 0.85 Urocyon minicephalus 0.96 1 Urocyon citronus 1 Urocyon galushi 0.96 Urocyon webbi 1 1 Metalopex merriami 0.23 Metalopex macconnelli 0.68 Leptocyon vafer 1 Leptocyon leidyi Leptocyon vulpinus 0.7 Leptocyon gregorii 1 Leptocyon douglassi Leptocyon mollis 0.32 Borophagus dudleyi 1 Borophagus hilli 0.98 Borophagus diversidens 0.97 Borophagus secundus 0.18 Borophagus parvus 1 Borophagus pugnator 1 Borophagus orc 1 Borophagus littoralis 0.96 Epicyon haydeni 1 0.92 Epicyon saevus Epicyon aelurodontoides Protepicyon raki 0.98 0.28 Carpocyon limosus 0.96 Carpocyon webbi 0.97 0.98 Carpocyon robustus Carpocyon compressus 0.17 0.87 Paratomarctus euthos Paratomarctus temerarius Tomarctus brevirostris 0.31 0.85 0.35 Tomarctus hippophaga 0.17 Tephrocyon rurestris Protomarctus opatus 0.24 0.58 Microtomarctus conferta Metatomarctus canavus Psalidocyon marianae 0.79 1 Aelurodon taxoides 0.32 0.27 Aelurodon ferox 1 Aelurodon stirtoni 0.14 Aelurodon mcgrewi 0.33 Aelurodon asthenostylus -

Murray-The Problem of Faunal Lists
THE PROBLEM OF FAUNAL LISTS Lyndon K Murray 1980 Anza-Borrego Desert State Park 1995 1968 2006 Live 1987 2004 1994 &1998 2008 Primary Faunal List • A list of taxa present in a stratigraphic context --used in-- – Institutional List - general information – Biostratigraphy List - local, restricted stratigraphic context, range of geologic ages – Biochronology List - Compilation of multiple localities over large geographic range, of same geologic age Types of Faunal Lists INSTITUTIONAL Report Venue Source Style Target • Web-site • In-house • Scientific • Public • Brochures lists • Informal • Non-science • Local news • Catalogue • Hyperbolic • Administration stories • Database • Wishful • Archive • Interpretive • Specimen thinking tour guides Labels • Internal • Field notes reports Types of Faunal Lists BIOSTRATIGRAPHY Report Venue Source Style Target • Professional • Catalogue • Formal • Professional journal • Database systematics • Scientists • Commercial • Specimen • Informal • Administration book Labels (qualifiers • Archive • Internal • Field may be report Notes stripped in text and figures) Types of Faunal Lists BIOCHRONOLOGY Report Venue Source Style Target • Edited book • Prior • Mixed • Professional • Website publications formats • Scientists (Faunmap, • Databases based on Paleobiology • Website published Database) (FaunMap, formats Paleobiology • Modified Database) published • Personal formats Communication Publication Venue Lowest Ranked Authority – In-house, not published – Report, institutional, not published, limited access – -
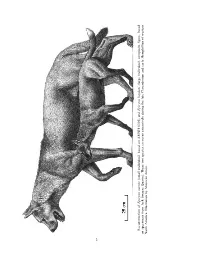
File # 01Acc (Large Individual, Composite figure, Based Epicyon Haydeni «N
AMNH BULLETIN Monday Oct 04 02:19 PM 1999 amnb 99111 Mp 2 Allen Press • DTPro System File # 01acc (large individual, composite figure, based Epicyon haydeni ´n. (small individual, based on AMNH 8305) and Epicyon saevus Reconstruction of on specimens from JackNorth Swayze America. Quarry). Illustration These by two Mauricio species Anto co-occur extensively during the late Clarendonian and early Hemphillian of western 2 1999 WANG ET AL.: SYSTEMATICS OF BOROPHAGINAE 3 CONTENTS Abstract ..................................................................... 9 Introduction .................................................................. 10 Institutional Abbreviations ................................................... 11 Acknowledgments .......................................................... 12 History of Study .............................................................. 13 Materials and Methods ........................................................ 18 Scope ...................................................................... 18 Species Determination ........................................................ 18 Taxonomic Nomenclature ..................................................... 19 Format ..................................................................... 19 Chronological Framework .................................................... 20 Definitions .................................................................. 21 Systematic Paleontology ....................................................... 21 Subfamily Borophaginae Simpson, -

Selections from Papers of the MASAL--Vol. 43 – Page 1 of 10
SUMMARY OF NORTH AMERICAN summaries of the Pleistocene fossil record in North America. A brief discussion of the problems PLEISTOCENE MAMMALIAN LOCAL FAUNAS encountered in age assignment of local faunas and use of provincial age and stage terms as applied to the CLAUDE W. HIBBARD Pleistocene follows. An explanation is given of the University of Michigan stratigraphic assignment of the local faunas in Table I. PAPERS OF THE MICHIGAN ACADEMY OP SCIENCE, ARTS, AND LETTERS VOL. XLIII, 1958 (1957 MEETING) Printed in U.S.A. DIFFERENT CONCEPTS OF THE PLEISTOCENE INTRODUCTION Zeuner (1950, p. 126) wrote, “the concept of Pleistocene THE ages of various Pleistocene faunas have plagued time has developed along three almost independent geologists since the first recognition of glacial and lines.” I list and briefly discuss these three different interglacial intervals. The chief handicap in assigning concepts here, since few students outside of the field of Pleistocene faunas to different ages is the lack of faunas vertebrate paleontology are aware of these problems. from the glaciated regions of North America which could Typology—Lyell (1833) named certain units of Cenozoic provide controls for dating faunas from the nonglaciated rocks Older Pliocene and Newer Pliocene. He later regions. Faunas are unknown from tills, and only a few (1839) applied the name Pliocene to what he previously fossils are known from outwash gravel and sand or from called “Older Pliocene” and the name Pleistocene to the interglacial deposits in the glaciated regions. With the “Newer Pliocene.” Calabrian and Villafranchian exception of post-Sangamon deposits, no vertebrate fossiliferous beds are present in the type section of fauna represented by more than a few specimens has Lyell’s Pliocene. -

Paleoecology of Fossil Species of Canids (Canidae, Carnivora, Mammalia)
UNIVERSITY OF SOUTH BOHEMIA, FACULTY OF SCIENCES Paleoecology of fossil species of canids (Canidae, Carnivora, Mammalia) Master thesis Bc. Isabela Ok řinová Supervisor: RNDr. V ěra Pavelková, Ph.D. Consultant: prof. RNDr. Jan Zrzavý, CSc. České Bud ějovice 2013 Ok řinová, I., 2013: Paleoecology of fossil species of canids (Carnivora, Mammalia). Master thesis, in English, 53 p., Faculty of Sciences, University of South Bohemia, České Bud ějovice, Czech Republic. Annotation: There were reconstructed phylogeny of recent and fossil species of subfamily Caninae in this study. Resulting phylogeny was used for examining possible causes of cooperative behaviour in Caninae. The study tried tu explain evolution of social behavior in canids. Declaration: I hereby declare that I have worked on my master thesis independently and used only the sources listed in the bibliography. I hereby declare that, in accordance with Article 47b of Act No. 111/1998 in the valid wording, I agree with the publication of my master thesis in electronic form in publicly accessible part of the STAG database operated by the University of South Bohemia accessible through its web pages. Further, I agree to the electronic publication of the comments of my supervisor and thesis opponents and the record of the proceedings and results of the thesis defence in accordance with aforementioned Act No. 111/1998. I also agree to the comparison of the text of my thesis with the Theses.cz thesis database operated by the National Registry of University Theses and a plagiarism detection system. In České Bud ějovice, 13. December 2013 Bc. Isabela Ok řinová Acknowledgements: First of all, I would like to thank my supervisor Věra Pavelková for her great support, patience and many valuable comments and advice. -

A New Genus and Species of Heron (Aves: Ardeidae) from the Late Miocene of Florida
A NEW GENUS AND SPECIES OF HERON (AVES: ARDEIDAE) FROM THE LATE MIOCENE OF FLORIDA David W. Steadman1 and Oona M. Takano2 ABSTRACT From the recently discovered Montbrook locality, Levy County, Florida (late Miocene; late Hemphillian land mammal age), a complete coracoid and nearly complete scapula represent a large heron that we name Taphophoyx hodgei new genus and species. While the phylogenetic affinities of T. hodgei are not well resolved, the tiger-herons Tigrisoma spp. or boat-billed heron Cochlearius cochlearius (both Neotropical) may be the closest living relative(s) of Taphophoyx, based in large part on several shared characters of the facies articularis clavicularis and facies articularis humeralis. Nevertheless, the coracoid of Taphophoyx has a uniquely prominent facies articularis humeralis and a uniquely sterno-ventral surface of corpus coracoidei. All 21 taxa of birds recorded thus far from Montbrook (mostly aquatic forms such as swans, ducks, geese, grebes, cormorants, ibises, sandpipers, etc.) probably represent extinct species, although Taphophoyx hodgei is the only one assigned to an extinct genus. Key words: Florida, Montbrook, late Miocene, heron, Ardeidae, new taxa. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank Publication number for this issue is BCAF821D-656A-4327- 95C1-F2F775F8A959. Published On-line: April 6, 2019 Open Access Download at https://www.floridamuseum.ufl.edu/bulletin/publications/ ISSN 2373-9991 Copyright © 2019 by the Florida Museum of Natural History, University of Florida. All rights reserved. Text, images and other media are for nonprofit, educational, or personal use of students, scholars, and the public.