3-Substituted-6-Aryl Pyridines As Ligands of C5a Receptors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aldrich FT-IR Collection Edition I Library
Aldrich FT-IR Collection Edition I Library Library Listing – 10,505 spectra This library is the original FT-IR spectral collection from Aldrich. It includes a wide variety of pure chemical compounds found in the Aldrich Handbook of Fine Chemicals. The Aldrich Collection of FT-IR Spectra Edition I library contains spectra of 10,505 pure compounds and is a subset of the Aldrich Collection of FT-IR Spectra Edition II library. All spectra were acquired by Sigma-Aldrich Co. and were processed by Thermo Fisher Scientific. Eight smaller Aldrich Material Specific Sub-Libraries are also available. Aldrich FT-IR Collection Edition I Index Compound Name Index Compound Name 3515 ((1R)-(ENDO,ANTI))-(+)-3- 928 (+)-LIMONENE OXIDE, 97%, BROMOCAMPHOR-8- SULFONIC MIXTURE OF CIS AND TRANS ACID, AMMONIUM SALT 209 (+)-LONGIFOLENE, 98+% 1708 ((1R)-ENDO)-(+)-3- 2283 (+)-MURAMIC ACID HYDRATE, BROMOCAMPHOR, 98% 98% 3516 ((1S)-(ENDO,ANTI))-(-)-3- 2966 (+)-N,N'- BROMOCAMPHOR-8- SULFONIC DIALLYLTARTARDIAMIDE, 99+% ACID, AMMONIUM SALT 2976 (+)-N-ACETYLMURAMIC ACID, 644 ((1S)-ENDO)-(-)-BORNEOL, 99% 97% 9587 (+)-11ALPHA-HYDROXY-17ALPHA- 965 (+)-NOE-LACTOL DIMER, 99+% METHYLTESTOSTERONE 5127 (+)-P-BROMOTETRAMISOLE 9590 (+)-11ALPHA- OXALATE, 99% HYDROXYPROGESTERONE, 95% 661 (+)-P-MENTH-1-EN-9-OL, 97%, 9588 (+)-17-METHYLTESTOSTERONE, MIXTURE OF ISOMERS 99% 730 (+)-PERSEITOL 8681 (+)-2'-DEOXYURIDINE, 99+% 7913 (+)-PILOCARPINE 7591 (+)-2,3-O-ISOPROPYLIDENE-2,3- HYDROCHLORIDE, 99% DIHYDROXY- 1,4- 5844 (+)-RUTIN HYDRATE, 95% BIS(DIPHENYLPHOSPHINO)BUT 9571 (+)-STIGMASTANOL -

Boranes: Physical & Chemical Properties, Encyclopaedia of Occupational Health and Safety, Jeanne Mager Stellman, Editor-In
Boranes: Physical & chemical properties, Encyclopaedia of Occupational Health and Safety, Jeanne Mager Stellman, Editor-in-Chief. International Labor Organization, Geneva. 2011. Chemical Name Colour/Form Boiling Point Melting Molecular Solubility in Relative Density Relative Vapour Inflam. Flash Auto CAS-Number (°C) Point (°C) Weight Water (water=1) Vapour Pressure/ Limits Point (°C) Ignition Density (Kpa) Point (°C) (air=1) BORON polymorphic: alpha- 2550 2300 10.81 insol Amorphous, 1.56x 580 3 -5 7440-42-8 rhombohedral form, clear 2.3 g/cm ; 10 red crystals; beta- alpha-- @ 2140 °C rhombohedral form, black; rhombohedral, - alpha-tetragonal form, 2.46 g/cm3; - black, opaque crystals with alpha-- metallic luster; amorphous tetragonal, - form, black or dark brown 2.31 g/cm3; - powder; other crystal beta-rhom- forms known bohedral, - 2.35 g/cm3 BORIC ACID, DISODIUM powder or glass-like 1575 741 201.3 2.56 g/100 g 2.367 SALT plates; white, free-flowing 1330-43-4 crystals; light grey solid BORON OXIDE rhombic crystals; 1860 450 69.6 2.77 g/100 g 1.8 1303-86-2 colourless, (amorphous); semitransparent lumps or 2.46 hard, white crystals (crystalline) BORON TRIBROMIDE colourless liquid 90 -46.0 250.57 reacts 2.6431 8.6 5.3 10294-33-4 @ 18.4 °C/4 °C @ 14 °C BORON TRICHLORIDE 12.5 -107 117.16 1.35 4.03 2.99 Pa 10294-34-5 @ 12 °C/4 @ 12.4 °C BORON TRIFLUORIDE colourless gas -99.9 -126.8 67.82 reacts 3.08g/1.57 l 2.4 10 mm Hg 7637-07-2 @ 4 °C @ -141 °C. -

Applications of Boronic Acids in Organic Synthesis
Applications of Boronic Acids in Organic Synthesis A dissertation presented by Pavel Starkov in partial fulfilment of the requirements for the award of the degree of DOCTOR OF PHILOSOPHY at UNIVERSITY COLLEGE LONDON Department of Chemistry Christopher Ingold Laboratories University College London 20 Gordon Street WC1H 0AJ London Declaration This dissertation is the result of my own work. Where information has been derived from other sources it has been clearly indicated so and acknowledged accordingly. /Pavel Starkov/ ii Abstract This thesis describes progress on the application of boronic acids and borate esters as catalysts and reagents in synthetic organic synthesis, focusing on two areas: one-pot enolate formation/aldol reactions and amide bond formation. Chapter 1 introduces the reader to boronic acids and derivatives thereof, their methods of preparation and their use in synthetic organic chemistry as reactants, reagents and catalysts. Chapter 2 covers current chemical methods and cellular alternatives for amide bond formation. Here, we also discuss our use of boron reagents for the activation of carboxylic acids as well as amides. Chapter 3 introduces a new concept in catalytic aldol reactions, i.e. an alternative strategy to access boron enolates in situ. The work covers successful demonstration of the feasibility of such an approach on an intramolecular system. A novel variation of aerobic Chan–Evans– Lam coupling, an intramolecular coupling of an aliphatic alcohol with a boronic acid using catalytic copper, is also introduced Chapter 4 builds on our observations on gold catalysis and especially that in relation to electrophilic halogenations. Chapter 5 contains full details of the experimental procedures. -

Boronic Acids
Boronic Acids Boronic Acids www.alfa.com INCLUDING: • Boronic Esters • Oxazaborolidine Reagents • Coupling and Hydroboration Catalysts • Phosphine Ligands • Borylation Reagents www.alfa.com Where Science Meets Service Quality Boronic Acids from Alfa Aesar Alfa Aesar is known worldwide for a variety of chemical compounds used in research and development. Recognized for purity and quality, our products and brands are backed by technical and sales teams dedicated to providing you the best service possible. In this catalog, you will find details on our line of boronic acids, esters and related compounds, which are manufactured to the same exacting standards as our full offering of over 33,000 products. Also included in this catalog is a 28-page introduction to boronic acids, their properties and applications. This catalog contains only a selection of our wide range of chemicals and materials. Also included is a selection of novel coupling catalysts and ligands. Many more products, including high purity metals, analytical products, and labware are available in our main catalog or online at www.alfa.com. Table of Contents About Us _____________________________________________________________________________ II How to Order/General Information ____________________________________________________ III Introduction __________________________________________________________________________ 1 Alkenylboronic acids and esters _____________________________________________________ 29 Alkylboronic acids and esters ________________________________________________________ -

Warwick.Ac.Uk/Lib-Publications
A Thesis Submitted for the Degree of PhD at the University of Warwick Permanent WRAP URL: http://wrap.warwick.ac.uk/139891 Copyright and reuse: This thesis is made available online and is protected by original copyright. Please scroll down to view the document itself. Please refer to the repository record for this item for information to help you to cite it. Our policy information is available from the repository home page. For more information, please contact the WRAP Team at: [email protected] warwick.ac.uk/lib-publications 1 AN INVESTIGATION OF SOME REACTIONS OF ALUMINIUM HYDROBORATE A thesis submitted for the degree of Doctor of Philosophy by David L.S. Shaw, B.Sc. The Department of Molecular Sciences^ University of Warwick 1. CONTENTS gage Contents 1 List of Tables 6 List of Figures 7 Acknowledgements 9 Sum m ary 10 1. Introduction 11 Nomenclature 11 Units 12 Hydroborates - Historical 12 Hydroborates - Properties 13 Hydroborates - Structures 17 Hydroborates - Bonding 21 Hydroborates - Vibrational Spectroscopy 24 Hydroborates - Nuclear Magnetic Spectral Properties 27 Aluminium Hydroborate 32 Preparation 32 Synthetic Reactions using Aluminium Hydroborate - Uses 33 Aluminium Hydroborate - Structure and Bonding 34 Aluminium Hydroborate - Properties 38 Physical Properties 38 Aluminium Hydroborate - Reactions 38 Exchange Reactions and Substituted Products 40 Anionic Aluminium Hydroborate Compounds 46 Adducts of Aluminium Hydroborates 47 Vibrational Spectra of Aluminium Hydroborate 50 Nuclear Magnetic Resonance of Aluminium Hydroborates 55 Decomposition of Aluminium Hydroborate - Hydride Aluminium Hydroborates 6-1 I Contents (continued ) Page Aluminium Hydride 63 Aluminium Alkyls 64 Aluminium and Higher Hydroborate Derivatives - Octahydrotriborates - 65 Trialkyl Boranes( Alkyl Diboranes 68 2. -

Nicolet Vapor Phase
Nicolet Vapor Phase Library Listing – 8,654 spectra This library is one the most comprehensive collections of vapor phase FT-IR spectra. It is an invaluable tool for scientist involved in investigations on gas phase materials. The Nicolet Vapor Phase Library contains 8654 FT-IR spectra of compounds measured in gas phase. Most spectra were acquired by the Sigma-Aldrich Co. using product samples. Additional spectra were collected by Hannover University, University of Wurzburg and Thermo Fisher Scientific applications scientists. Spectra were collected using sampling techniques including heated or room temperature gas cell or a heated light-pipe connected to the outlet of a gas chromatograph. Nicolet Vapor Phase Index Compound Name Index Compound Name 8402 ((1- 5457 (-)-8-Phenylmenthol; (-)-(1R,2S,5R)-5- Ethoxycyclopropyl)oxy)trimethylsilane Methyl-2-(2-phenyl-2-propyl)cyc 4408 (+)-1,3-Diphenylbutane 1095 (-)-Carveol, mixture of isomers; p- 4861 (+)-1-Bromo-2,4-diphenylbutane Mentha-6,8-dien-2-ol 2406 (+)-3-(Heptafluorobutyryl)camphor 3628 (-)-Diisopropyl D-tartrate 2405 (+)-3-(Trifluoroacetyl)camphor 1427 (-)-Limonene oxide, cis + trans; (-)-1,2- 281 (+)-3R-Isolimonene, trans-; (1R,4R)- Epoxy-4-isopropenyl-1-methyl (+)-p-Mentha-2,8-diene 1084 (-)-Menthol; [1R-(1a,2b,5a)]-(-)-2- 289 (+)-Camphene; 2,2-Dimethyl-3- Isopropyl-5-methylcyclohexanol methylenebicyclo[2.2.1]heptane 2750 (-)-Menthoxyacetic acid 3627 (+)-Diisopropyl L-tartrate 1096 (-)-Myrtanol, cis-; (1S,2R)-6,6- 2398 (+)-Fenchone; (+)-1,3,3- Dimethylbicyclo[3.1.1]heptane-2-metha -

Electrosynthesis of Lithium Borohydride from Trimethyl Borate and Hydrogen Gas
Electrosynthesis of Lithium Borohydride from Trimethyl Borate and Hydrogen Gas By James Mokaya Omweri Submitted in Partial Fulfillment of the Requirements For the Degree of Master of Science In the Chemistry Program YOUNGSTOWN STATE UNIVERSITY December, 2019 Electrosynthesis of Lithium Borohydride from Trimethyl Borate and Hydrogen Gas James Mokaya Omweri I hereby release this thesis to the public. I understand that this thesis will be made available from the OhioLINK ETD Center and the Maag Library Circulation Desk for public access. I also authorize the University or other individuals to make copies of this thesis as needed for scholarly research. Signature: James Mokaya Omweri, Student Date Approvals: Dr. Clovis A. Linkous, Thesis Advisor Date Date Dr. Sherri Lovelace-Cameron, Committee Member Date Dr. Timothy Wagner, Committee Member Date Dr. Salvatore A. Sanders, Dean of Graduate Studies Date ABSTRACT Solid state hydrogen storage is seen as the ultimate answer in realizing the hydrogen economy and minimizing our overdependence on nonrenewable fossil fuels. The use of fossil fuels has contributed negatively towards climate change, leading to a lot of future uncertainties. Alkali metal borohydrides, especially lithium borohydride, with desirable H2 storage properties such dry air stability and high gravimetric and volumetric storage densities of 18.5 wt% and 121 kg/m3, respectively, are proving to be some of the most important solid state hydrogen storage materials. However, their current cost of production is very high considering that most borohydrides are synthesized from sodium borohydride, which in turn is made from sodium hydride, NaH, and trimethyl borate, B(OCH3)3. NaH, which is a product of an energetic process, namely hydrogenation following electrodeposition of the metal from a molten salt, acts as the source of H- required for the formation of the borohydride ion. -

Organic Compounds of Boron M
ORGANIC COMPOUNDS OF BORON M. F. LAPPERT Chemistry Department, Northern Polytechnic, Holloway, London, N. 7, England Received January 18, 1866 CONTENTS I. Introduction. 961 963 A, Introduction. .......I.............. 963 963 1. Introductio ... I............ 963 2. Boron trich 964 .................... 965 965 ...I....... 966 966 967 967 of orthoborates.. , . , . 968 s of orthoborates . , . , . , , . 971 1. Thermal stability. , . 971 2. Solvolysis reactions and reactions with acids. , . , . , . , . , . , . , . 971 3. Halogenation reactions.. t.. , . , , . , , . 972 4. Reaction with ammonia and amines. , . , . , , . 973 5. Borate complexes with metal salts. , . , . 974 6. Other reactions. , . , . , . , , , . , . , , , , , 976 E. Chelated orthoborates and borates of polyhydric alcohols. t.. 976 F. Mixed orthoborates . , . 979 G. Acyl borates.. , . , . , . , . , . , . , , . , , 980 H. Metaborates . , . , . , . 982 I. Thioorthoborates. , . t. , . , . , . , . , . 983 111. Derivatives of HB(OH)* and HBO 983 A. Introduction. , . 983 B. The dialkoxyborines . , . , . , , . , . , . , . , . , , , 984 C. Preparation of boronic acids and esters. , , . , , , , . , . , 985 1. Introduction. , . 985 2. Oxidation of trialkylborons . , . , , . , , , . , , 985 3. Metal alkyls or aryls on trialkyl borates.. , , , . 985 4. Hydrolysis or alcoholysis of alkyl- or arylboron dihalides (produced by the action of metal alkyls 986 5. Other methods for boronic a ........... 987 6. Special methods for boronic .................................... 988 D. Physical properties -
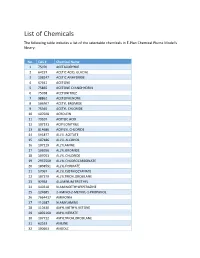
List of Chemicals the Following Table Includes a List of the Selectable Chemicals in E‐Plan Chemical Plume Model's Library
List of Chemicals The following table includes a list of the selectable chemicals in E‐Plan Chemical Plume Model's library: No. CAS # Chemical Name 1 75070 ACETALDEHYDE 2 64197 ACETIC ACID, GLACIAL 3 108247 ACETIC ANHYDRIDE 4 67641 ACETONE 5 75865 ACETONE CYANOHYDRIN 6 75058 ACETONITRILE 7 98862 ACETOPHENONE 8 506967 ACETYL BROMIDE 9 75365 ACETYL CHLORIDE 10 107028 ACROLEIN 11 79107 ACRYLIC ACID 12 107131 ACRYLONITRILE 13 814686 ACRYLYL CHLORIDE 14 591877 ALLYL ACETATE 15 107186 ALLYL ALCOHOL 16 107119 ALLYLAMINE 17 106956 ALLYL BROMIDE 18 107051 ALLYL CHLORIDE 19 2937500 ALLYL CHLOROCARBONATE 20 1838591 ALLYL FORMATE 21 57067 ALLYL ISOTHIOCYANATE 22 107379 ALLYLTRICHLOROSILANE 23 97938 ALUMINUM TRIETHYL 24 140318 N‐AMINOETHYLPIPERAZINE 25 124685 2‐AMINO‐2‐METHYL‐1‐PROPANOL 26 7664417 AMMONIA 27 110587 N‐AMYLAMINE 28 110430 AMYL METHYL KETONE 29 1002160 AMYL NITRATE 30 107722 AMYLTRICHLOROSILANE 31 62533 ANILINE 32 100663 ANISOLE No. CAS # Chemical Name 33 7647189 ANTIMONY PENTACHLORIDE 34 7783702 ANTIMONY PENTAFLUORIDE 35 7784341 ARSENIC TRICHLORIDE 36 7784421 ARSINE 37 71432 BENZENE 38 100470 BENZONITRILE 39 98077 BENZOTRICHLORIDE 40 98088 BENZOTRIFLUORIDE 41 98884 BENZOYL CHLORIDE 42 100469 BENZYLAMINE 43 100390 BENZYL BROMIDE 44 100447 BENZYL CHLORIDE 45 501531 BENZYL CHLOROFORMATE 46 98873 BENZYLIDENE CHLORIDE 47 10294334 BORON TRIBROMIDE 48 10294345 BORON TRICHLORIDE 49 7637072 BORON TRIFLUORIDE 50 109637 BORON TRIFLUORIDE DIETHYL ETHERATE 51 353424 BORON TRIFLUORIDE DIMETHYL ETHERATE 52 7726956 BROMINE 53 13863417 BROMINE CHLORIDE 54 7789302 BROMINE PENTAFLUORIDE 55 7787715 BROMINE TRIFLUORIDE 56 598312 BROMOACETONE 57 108861 BROMOBENZENE 58 109659 1‐BROMOBUTANE 59 353593 BROMOCHLORODIFLUOROMETHANE 60 74975 BROMOCHLOROMETHANE 61 540512 2‐BROMOETHANOL 62 75252 BROMOFORM 63 107824 1‐BROMO‐3‐METHYLBUTANE 64 75638 BROMOTRIFLUOROMETHANE 65 106990 1,3‐BUTADIENE 66 106978 BUTANE 67 106989 1‐BUTENE 68 590181 2‐BUTENE‐CIS 69 624646 2‐BUTENE‐TRANS 70 123864 BUTYL ACETATE 71 105464 SEC‐BUTYL ACETATE No. -

Durham E-Theses
Durham E-Theses Studies on the organic chemistry of boron Livingstone, J.G. How to cite: Livingstone, J.G. (1961) Studies on the organic chemistry of boron, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/9111/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk (11 ft u 4 SIAO rsi ^> V 3^NVJ-i3>Vc)V3 Nl 39IMVH3 0 6> CM 5 li 70 7D 0 0> 6 ID 8 79 730 Uoi 0 STUDIES ON THE ORGANIC CHEMISTRY OF BORON BY J. Gr« LIVINGSTONE A dissertation submitted for the Degree of Doctor of Philosophy in the Durham Colleges of the University of Durham. 1961 CONTENTS Page Acknowledgments (1) Memorandum (II) Summary (Ill) FART I Introduction Page Organor-Derivatives of Boric Acid I Trialkyl- and Triaryl Boranes 8 Mixed Trialkyl and Aryl-Alkylboranes 40 Cyclic Boranes 43 Boronic Acids and Esters 46 Borinic Acids and Esters 54 Boroxines 62 Alkyl- and Aryl- Dihaloboranes 65 Dialkyl. -

Synthesis and Reactions Of
SYNTHESIS AND REACTIONS OF HETEROSUBSTITUTED N-HETEROCYCLIC CARBENE-BORANES by Everett William Merling B.S., Xavier University, 2009 Submitted to the Graduate Faculty of the Kenneth P. Dietrich School of Arts and Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 2015 UNIVERSITY OF PITTSBURGH DIETRICH SCHOOL OF ARTS AND SCIENCES This dissertation was presented by Everett William Merling It was defended on April 10, 2015 and approved by Professor Theodore Cohen, Department of Chemistry Professor Scott Nelson, Department of Chemistry Professor Yadong Wang, Department of Bioengineering Committee Chair: Professor Dennis P. Curran, Department of Chemistry ii Copyright © by Everett William Merling 2015 iii SYNTHESIS AND REACTIONS OF HETEROSUBSTITUTED N-HETEROCYCLIC CARBENE-BORANES Everett William Merling, PhD University of Pittsburgh, 2015 Carbene-boranes 1,3-bis-(2,6-diisopropylphenyl)imidazol-2-ylidene borane (dipp-Imd- BH3) and 1,3-dimethylimidazol-2-ylidene borane (diMe-Imd-BH3) were treated with various electrophiles to compare reactivity of carbene-borane with nucleophilicity values of carbene- borane (dipp-Imd-BH3 = 9.55 N, diMe-Imd-BH3 = 11.88 N). Compounds with electrophilicity values greater –15 E reacted rapidly with carbene-borane, but compounds with electrophilicity values less than –15 E reacted slowly or not at all with carbene-borane. Hydroboration complexes were isolated from the reactions of diMe-Imd-BH3 with benzylidenemalononitrile and 2-ethylidenemalononitrile. Overall the reactivity of carbene-borane matched predictions based on nucleophilicity and electrophilicity values. Carbene-boranes were also treated with acid halides and halogens to form halogenated carbene-boranes. Iodination occurs most rapidly followed by bromination and then chlorination. -

Physical Properties of Chemicals in PAC Revision 27 Listing
LLNL-TR-625492 Physical Properties of Chemicals in PAC Revision 27 Listing M. A. Johnson March 8, 2013 Disclaimer This document was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor Lawrence Livermore National Security, LLC, nor any of their employees makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or Lawrence Livermore National Security, LLC. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or Lawrence Livermore National Security, LLC, and shall not be used for advertising or product endorsement purposes. This work performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. Physical Properties of Chemicals in PAC Revision 27 Listing 1 Purpose The purpose of this chemical physical property listing is to provide data required to apply the DOE SCAPA Protective Action Criteria (PAC) values to calculation of the LLNL Quantity (Q) Value thresholds for facility chemical hazard classification. This chemical physical property listing based on the DOE SCAPA Protective Action Criteria (PAC) Revision 27 listing Identifies: 1. Physical state at 25°C (i.e.