1 Radioactive Decay and the Origin Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stable Isotopes of Cobalt Properties of Cobalt
Stable Isotopes of Cobalt Isotope Z(p) N(n) Atomic Mass Natural Abundance Nuclear Spin Co-59 27 32 58.93320 100.00% 7/2- Cobalt was discovered in 1735 by Georg Brandt. Its name derives from the German word kobald, meaning "goblin" or "evil spirit." Minerals containing cobalt were used by the early civilizations of Egypt and Mesopotamia for coloring glass deep blue. Cobalt oxide is used today to add a pink or blue color to glass. It is also an important trace element in soils and necessary for animal nutrition. The most important modern use of cobalt is in the manufacture of various wear-resistant and superalloys. Its alloys have shown high resistance to corrosion and oxidation at high temperatures. Radioactive Cobalt-60 is used in radiography and in the sterilization of food. A silvery-white, shining, hard, ductile, somewhat malleable metal, cobalt is also ferromagnetic, with permeability two-thirds that of iron. It has exceptional magnetic properties in alloys. It is attached by dilute hydrochloric and sulfuric acids. It corrodes readily in air, and it has unusual coordinating properties, especially the trivalent ion. It is noncombustible except in powder form. Cobalt occurs in two allotropic modifications over a wide range of temperatures: the crystalline close-packed- hexagonal form is known as the alpha form, which turns into the beta (or gamma) form above 417 ºC. In finely powdered form, cobalt ignites spontaneously in air. Reactions with acetylene and bromine pentafluoride proceed to incandescence and can become violent. The metal is moderately toxic by ingestion. Inhalation of dusts can damage lungs. -

Annual Report 1951 National Bureau of Standards
Annual Report 1951 National Bureau of Standards Miscellaneous Publication 204 UNITED STATES DEPARTMENT OF COMMERCE Charles Sawyer, Secretary NATIONAL BUREAU OF STANDARDS A. V. Astint, Director Annual Report 1951 National Bureau of Standards For sale by the Superintendent of Documents, U. S. Government Printing Office Washington 2 5, D. C. Price 50 cents CONTENTS Page 1. General Review 1 2. Electricity 16 Beam intensification in a high-voltage oscillograph 17, Low-temperature dry cells 17, High-rate batteries 17, Battery additives 18. 3. Optics and Metrology 18 The kinorama 19, Measurement of visibility for aircraft 20, Antisubmarine aircraft searchlights 20, Resolving power chart 20, Refractivity 21, Thermal expansivity of aluminum alloys 21. 4. Heat and Power . 21 Thermodynamic properties of materials 22, Synthetic rubber and other high polymers 23, Combustors for jet engines 24, Temperature and composition of flames 25, Engine "knock" 25, Low-temperature physics 26, Medical physics instrumentation 28. 5. Atomic and Radiation Physics 28 Atomic standard of length 29, Magnetic moment of the proton 29, Spectra of artificial elements 31, Photoconductivity of semiconductors 31, Radiation detecting instruments 32, Protection against radiation 32, X-ray equipment 33, Atomic and molecular ions 35, Electron physics 35, Tables of nuclear data 35, Atomic energy levels 36. 6. Chemistry 36 Radioactive carbohydrates 36, Dextran as a substitute for blood plasma 37, Acidity and basicity in organic solvents 37, Interchangeability of fuel gases 38, Los Angeles "smog" 39, Infrared spectra of alcohols 39, Electrodeposi- tion 39, Development of analytical methods 40, Physical constants 42. 7. Mechanics 42 Turbulent flow 43, Turbulence at supersonic speeds 43, Dynamic properties of materials 43, High-frequency vibrations 44, Hearing loss 44, Physical properties by sonic methods 44, Water waves 46, Density currents 46, Precision weighing 46, Viscosity of gases 46, Evaporated thin films 47. -

Chapter 2 Atoms, Molecules and Ions
Chapter 2 Atoms, Molecules and Ions PRACTICING SKILLS Atoms:Their Composition and Structure 1. Fundamental Particles Protons Electrons Neutrons Electrical Charges +1 -1 0 Present in nucleus Yes No Yes Least Massive 1.007 u 0.00055 u 1.007 u 3. Isotopic symbol for: 27 (a) Mg (at. no. 12) with 15 neutrons : 27 12 Mg 48 (b) Ti (at. no. 22) with 26 neutrons : 48 22 Ti 62 (c) Zn (at. no. 30) with 32 neutrons : 62 30 Zn The mass number represents the SUM of the protons + neutrons in the nucleus of an atom. The atomic number represents the # of protons, so (atomic no. + # neutrons)=mass number 5. substance protons neutrons electrons (a) magnesium-24 12 12 12 (b) tin-119 50 69 50 (c) thorium-232 90 142 90 (d) carbon-13 6 7 6 (e) copper-63 29 34 29 (f) bismuth-205 83 122 83 Note that the number of protons and electrons are equal for any neutral atom. The number of protons is always equal to the atomic number. The mass number equals the sum of the numbers of protons and neutrons. Isotopes 7. Isotopes of cobalt (atomic number 27) with 30, 31, and 33 neutrons: 57 58 60 would have symbols of 27 Co , 27 Co , and 27 Co respectively. Chapter 2 Atoms, Molecules and Ions Isotope Abundance and Atomic Mass 9. Thallium has two stable isotopes 203 Tl and 205 Tl. The more abundant isotope is:___?___ The atomic weight of thallium is 204.4 u. The fact that this weight is closer to 205 than 203 indicates that the 205 isotope is the more abundant isotope. -
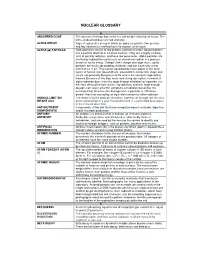
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Załącznik Nr 3 Do Wniosku O Przeprowadzenie Postępowania Habilitacyjnego
Załącznik nr 3 do wniosku o przeprowadzenie postępowania habilitacyjnego Author's review of his own writings 1. Jan Kurpeta 2. Posiadane dyplomy, stopnie naukowe Degree of Doctor of Philosophy in physics, University of Warsaw, Faculty of Physics, year 1999, title of dissertation: „Properties of the neutron-rich nuclei at the edge of known nuclei area.” Master of Science and Engineering, Warsaw University of Technology, Faculty of Technical Physics and Applied Mathematics, year 1993, title of master's thesis: „Influence of nucleon effective mass on single particle motion in the deformed nuclei.” 3. Informacje o dotychczasowym zatrudnieniu w jednostkach naukowych University of Warsaw, Faculty of Physics, Institute of Experimental Physics, Nuclear Spectroscopy Division, assistant professor since15 February 2001 University of Leuven (Belgium), Instituut voor Kern- en Stralingsfysica, post-doc position from 1 September 1998 to 31 October 1999 University of Jyväskylä (Finland), Faculty of Physics, scholarship from Center for International Mobility, PhD studies from 3 February to 15 December 1995 University of Warsaw, Faculty of Physics, Institute of Experimental Physics, Nuclear Spectroscopy Division, PhD studies from October 1993 to September 1998 Space Research Center Polish Academy of Sciences in Warsaw, Department of Planetary Geodesy, part time job for 6 months in 1993 1 4. Wskazanie osiągnięcia* wynikającego z art. 16 ust. 2 ustawy z dnia 14 marca 2003 r. o stopniach naukowych i tytule naukowym oraz o stopniach i tytule w zakresie sztuki (Dz. U. nr 65, poz. 595 ze zm.): a) tytuł osiągnięcia naukowego Structure of exotic, neutron-rich fission fragments of mass around A = 110. b) Jednotematyczny cykl publikacji przedstawiających osiągnięcie naukowe [A1] J. -

Introduction to Nuclear Physics and Nuclear Decay
NM Basic Sci.Intro.Nucl.Phys. 06/09/2011 Introduction to Nuclear Physics and Nuclear Decay Larry MacDonald [email protected] Nuclear Medicine Basic Science Lectures September 6, 2011 Atoms Nucleus: ~10-14 m diameter ~1017 kg/m3 Electron clouds: ~10-10 m diameter (= size of atom) water molecule: ~10-10 m diameter ~103 kg/m3 Nucleons (protons and neutrons) are ~10,000 times smaller than the atom, and ~1800 times more massive than electrons. (electron size < 10-22 m (only an upper limit can be estimated)) Nuclear and atomic units of length 10-15 = femtometer (fm) 10-10 = angstrom (Å) Molecules mostly empty space ~ one trillionth of volume occupied by mass Water Hecht, Physics, 1994 (wikipedia) [email protected] 2 [email protected] 1 NM Basic Sci.Intro.Nucl.Phys. 06/09/2011 Mass and Energy Units and Mass-Energy Equivalence Mass atomic mass unit, u (or amu): mass of 12C ≡ 12.0000 u = 19.9265 x 10-27 kg Energy Electron volt, eV ≡ kinetic energy attained by an electron accelerated through 1.0 volt 1 eV ≡ (1.6 x10-19 Coulomb)*(1.0 volt) = 1.6 x10-19 J 2 E = mc c = 3 x 108 m/s speed of light -27 2 mass of proton, mp = 1.6724x10 kg = 1.007276 u = 938.3 MeV/c -27 2 mass of neutron, mn = 1.6747x10 kg = 1.008655 u = 939.6 MeV/c -31 2 mass of electron, me = 9.108x10 kg = 0.000548 u = 0.511 MeV/c [email protected] 3 Elements Named for their number of protons X = element symbol Z (atomic number) = number of protons in nucleus N = number of neutrons in nucleus A A A A (atomic mass number) = Z + N Z X N Z X X [A is different than, but approximately equal to the atomic weight of an atom in amu] Examples; oxygen, lead A Electrically neural atom, Z X N has Z electrons in its 16 208 atomic orbit. -

High Specific Radioactivities of Cobalt, Platinum and Iridium From
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1951 High specific ar dioactivities of cobalt, platinum and iridium from photonuclear reactions Darleane Christian Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Physical Chemistry Commons Recommended Citation Christian, Darleane, "High specific ar dioactivities of cobalt, platinum and iridium from photonuclear reactions " (1951). Retrospective Theses and Dissertations. 13387. https://lib.dr.iastate.edu/rtd/13387 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. NOTE TO USERS This reproduction is the best copy available. UMI HIGH SPECIFIC MDIOACTIVITI OF COBALT, PLATIirOl-I Mm IRIDIUIl FROM PHOTOirUCL:mR R3ACTI0KS by Darloana Christian A Dissertation S-ubmitted to the Graduate Faculty in Partial Fulfillmant of The Requiramants for the Degrea of DOCTOR OP PHILOflOPIir Major Subject: Physical Chamistry Approveds Signature was redacted for privacy. In Cha3?i¥ of tlay6r' Worls: Signature was redacted for privacy. Heaa of tiajor Departraent ^ Signature was redacted for privacy. Daan of Graduate Collage loi/a State College 1951 UMI Number: DP12638 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. -

Comments on Evaluation of Al Electron-Capture and Positron
26 Comments on evaluation – CEA ISBN 2 7272 0211 3 Al Comments on evaluation of 26Al Electron-Capture and Positron Decay Data by Shiu-Chin Wu and E. Browne The Limitation of Relative Statistical Weight [2] (LWM) method, used for averaging numbers throughout this evaluation, provided a uniform approach for the analysis of discrepant data. The uncertainty assigned to the recommended values was always greater than or equal to the smallest uncertainty of the values used to calculate the average. Decay Scheme 26Al decays 100% by EC + b+ to 26Mg 2+ states at 1808.72 and 2938.41 keV. A measured relative emission probability for the positron annihilation radiation (511 keV) produced a total b+ branching of 82(2)% (72Sa02). This branching agrees well with a value of 81.7% predicted by theory[1]. Nuclear Data The recommended half-life of 26Al, 7.17(24) ´ 105 y, is a weighted average (c2/N-1=0.64, LWM) of 5 + 5 + 7.16(32) ´ 10 y, from partial T1/2 (b )= 8.73(30) ´ 10 y, b counting and mass spectrometric analysis [3], combined with b+(%)=82(2) from measurement of positron annihilation radiation (72Sa02)); 7.05(24) ´ 105 y, by specific activity and mass spectrometric analysis [4]; 7.8 (5) ´ 105 y, by specific activity from a source produced by 26Mg(p,n) 26Al (the number of 26Al atoms in the source were estimated by using the reaction cross section) [5]; and 7.02 (56) ´ 105 y, by counting the atoms of 26Al that did not disintegrate in the source (Accelerator Mass Spectrometry) [6]. -

Radioactivity and X-Rays
Radioactivity and X-rays Applications and health effects by Thormod Henriksen Preface The present book is an update and extension of three previous books from groups of scientists at the University of Oslo. The books are: I. Radioaktivitet – Stråling – Helse Written by; Thormod Henriksen, Finn Ingebretsen, Anders Storruste and Erling Stranden. Universitetsforlaget AS 1987 ISBN 82-00-03339-2 I would like to thank my coauthors for all discussions and for all the data used in this book. The book was released only a few months after the Chernobyl accident. II. Stråling og Helse Written by Thormod Henriksen, Finn Ingebretsen, Anders Storruste, Terje Strand, Tove Svendby and Per Wethe. Institute of Physics, University of Oslo 1993 and 1995 ISBN 82-992073-2-0 This book was an update of the book above. It has been used in several courses at The University of Oslo. Furthermore, the book was again up- dated in 1998 and published on the Internet. The address is: www.afl.hitos.no/mfysikk/rad/straling_innh.htm III. Radiation and Health Written by Thormod Henriksen and H. David Maillie Taylor & Francis 2003 ISBN 0-415-27162-2 This English written book was mainly a translation from the books above. I would like to take this opportunity to thank David for all help with the translation. The three books concentrated to a large extent on the basic properties of ionizing radiation. Efforts were made to describe the background ra- diation as well as the release of radioactivity from reactor accidents and fallout from nuclear explosions in the atmosphere. These subjects were of high interest in the aftermath of the Chernobyl accident. -

1. Public Health Statement
COBALT 1 1. PUBLIC HEALTH STATEMENT This public health statement tells you about cobalt and the effects of exposure. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites make up the National Priorities List (NPL) and are the sites targeted for long-term federal cleanup activities. Stable cobalt has been found in at least 426 of the 1,636 current or former NPL sites. Radioactive cobalt, as 60Co, has been found in at least 13 of the 1,636 current or former NPL sites. However, the total number of NPL sites evaluated for this substance is not known. As more sites are evaluated, the sites at which cobalt is found may increase. This information is important because exposure to this substance may harm you and because these sites may be sources of exposure. When a substance is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. This release does not always lead to exposure. You are exposed to a substance only when you come in contact with it. You may be exposed by breathing, eating, or drinking the substance, or by skin contact. External exposure to radiation may occur from natural or man-made sources. Naturally occurring sources of radiation are cosmic radiation from space or radioactive materials in soil or building materials. Man-made sources of radioactive materials are found in consumer products, industrial equipment, atom bomb fallout, and to a smaller extent from hospital waste and nuclear reactors. -

2008 Summerresidentlecture
Atoms Mass and Energy Units Nucleus: ~10-14 m diameter and Introduction to Nuclear ~1017 kg/m3 Mass-Energy Equivalence Electron clouds: ~10-10 m diameter Physics and Nuclear Decay (= size of atom) Mass water molecule: ~10-10 m diameter atomic mass unit, u (or amu): mass of 12C ≡ 12.000000 u ~103 kg/m3 1 u = 1.660540 x 10-27 kg = 931.494 x 106 eV/c2 Nucleons (protons and neutrons) are ~10,000 times Energy smaller than the atom, and ~1800 times more massive Larry MacDonald Electron volt, eV ≡ kinetic energy attained by an electron accelerated through 1.0 volt than electrons. 22 May 2008 (electron size < 10-22 m (only an upper limit can be estimated)) 1 eV = 1.6 x10-19 J 2 Nuclear and atomic units of length E = mc c = 3 x 108 m/s -15 10 = femtometer (fm) speed of light 10-10 = angstrom (Å) Molecules -27 2 Course website (Nuclear Medicine Imaging) mass of proton, mp = 1.6724x10 kg = 1.007276 u = 938.3 MeV/c -27 2 mostly empty space mass of neutron, mn = 1.6747x10 kg = 1.008655 u = 939.6 MeV/c http://depts.washington.edu/uwmip/ Hecht, Physics, 1994 -31 2 Water mass of electron, me = 9.108x10 kg = 0.000548 u = 0.511 MeV/c (wikipedia) 22 May 2008 2 22 May 2008 3 Elements Named for their number of protons Nuclide Groups/Families N vs. Z Chart of Nuclides X = element symbol Z (atomic number) = number of protons in nucleus N > Z for the majority A nuclide is a nucleus with a specific Z and A (N = Z for low Z elements) N = number of neutrons in nucleus A A A ~1500 nuclides exist A (atomic mass number) = Z + N Z X N Z X X [A is different than, but approximately equal to the atomic (Periodic Table typically lists distinct Z) The line of stability (gold band) weight of an atom] Examples; oxygen, lead represents the stable nuclei. -

Hacking the Atom
Hacking the Atom EXPLORATIONS IN NUCLEAR RESEARCH, VOL. 1 The new science of low-energy nuclear reactions (1990-2015) Steven B. Krivit Edited by Michael J. Ravnitzky SAN RAFAEL, CALIFORNIA Hacking the Atom: Explorations in Nuclear Research, Vol. 1 Copyright © 2016 by Steven B. Krivit All rights reserved. No part of this book may be reproduced in whole or in part without written permission from the publisher, except by reviewers who may quote brief excerpts in connection with a review in a newspaper, magazine, or electronic publication; nor may any part of this book be reproduced, stored in a retrieval system, or transmitted in any form or by any means electronic without permission from the publisher. Pacific Oaks Press / New Energy Times / www.newenergytimes.com 369-B 3rd St. #556, San Rafael, CA 94901 Library of Congress Control Number: 2016902974 Krivit, Steven B., author. Hacking the atom / Steven B. Krivit ; editors, Michael Ravnitzky, Cynthia Goldstein, Mat Nieuwenhoven. pages cm -- (Explorations in nuclear research ; vol. 1) Includes bibliographical references and index. LCCN 2016902974 ISBN 978-0-996886444 (hbk.) ISBN 978-0-996886451 (pbk.) ISBN 978-0-996886468 (Kindle) ISBN 978-0-996886475 (ePUB) 1. Low-energy nuclear reactions--Research--History. 2. Electroweak interactions--Research--History. 3. Science--Social aspects. I. Ravnitzky, Michael, editor. II. Goldstein, Cynthia, editor. III. Nieuwenhoven, Mat, editor. IV. Title. V. Series: Krivit, Steven B. Explorations in nuclear research ; v. 1. QC794.8.L69K754 2016 539.7'5 QBI16-600060 Cover design: Lucien G. Frisch (Photograph: © Jahoo | Dreamstime.com) Interior design template: Book Design Templates Inc. Typeset in Crimson 11 pt., designed by Sebastian Kosch Editors: Michael Ravnitzky (Developmental Editor), Cynthia Goldstein (Copy Editor), Mat Nieuwenhoven (Technical Editor) Index: Laura Shelly Index Page numbers in bold indicate figures, Baldwin, Richard S., 52 tables, and photos.