2008 Summerresidentlecture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
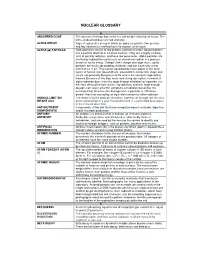
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Introduction to Nuclear Physics and Nuclear Decay
NM Basic Sci.Intro.Nucl.Phys. 06/09/2011 Introduction to Nuclear Physics and Nuclear Decay Larry MacDonald [email protected] Nuclear Medicine Basic Science Lectures September 6, 2011 Atoms Nucleus: ~10-14 m diameter ~1017 kg/m3 Electron clouds: ~10-10 m diameter (= size of atom) water molecule: ~10-10 m diameter ~103 kg/m3 Nucleons (protons and neutrons) are ~10,000 times smaller than the atom, and ~1800 times more massive than electrons. (electron size < 10-22 m (only an upper limit can be estimated)) Nuclear and atomic units of length 10-15 = femtometer (fm) 10-10 = angstrom (Å) Molecules mostly empty space ~ one trillionth of volume occupied by mass Water Hecht, Physics, 1994 (wikipedia) [email protected] 2 [email protected] 1 NM Basic Sci.Intro.Nucl.Phys. 06/09/2011 Mass and Energy Units and Mass-Energy Equivalence Mass atomic mass unit, u (or amu): mass of 12C ≡ 12.0000 u = 19.9265 x 10-27 kg Energy Electron volt, eV ≡ kinetic energy attained by an electron accelerated through 1.0 volt 1 eV ≡ (1.6 x10-19 Coulomb)*(1.0 volt) = 1.6 x10-19 J 2 E = mc c = 3 x 108 m/s speed of light -27 2 mass of proton, mp = 1.6724x10 kg = 1.007276 u = 938.3 MeV/c -27 2 mass of neutron, mn = 1.6747x10 kg = 1.008655 u = 939.6 MeV/c -31 2 mass of electron, me = 9.108x10 kg = 0.000548 u = 0.511 MeV/c [email protected] 3 Elements Named for their number of protons X = element symbol Z (atomic number) = number of protons in nucleus N = number of neutrons in nucleus A A A A (atomic mass number) = Z + N Z X N Z X X [A is different than, but approximately equal to the atomic weight of an atom in amu] Examples; oxygen, lead A Electrically neural atom, Z X N has Z electrons in its 16 208 atomic orbit. -

Comments on Evaluation of Al Electron-Capture and Positron
26 Comments on evaluation – CEA ISBN 2 7272 0211 3 Al Comments on evaluation of 26Al Electron-Capture and Positron Decay Data by Shiu-Chin Wu and E. Browne The Limitation of Relative Statistical Weight [2] (LWM) method, used for averaging numbers throughout this evaluation, provided a uniform approach for the analysis of discrepant data. The uncertainty assigned to the recommended values was always greater than or equal to the smallest uncertainty of the values used to calculate the average. Decay Scheme 26Al decays 100% by EC + b+ to 26Mg 2+ states at 1808.72 and 2938.41 keV. A measured relative emission probability for the positron annihilation radiation (511 keV) produced a total b+ branching of 82(2)% (72Sa02). This branching agrees well with a value of 81.7% predicted by theory[1]. Nuclear Data The recommended half-life of 26Al, 7.17(24) ´ 105 y, is a weighted average (c2/N-1=0.64, LWM) of 5 + 5 + 7.16(32) ´ 10 y, from partial T1/2 (b )= 8.73(30) ´ 10 y, b counting and mass spectrometric analysis [3], combined with b+(%)=82(2) from measurement of positron annihilation radiation (72Sa02)); 7.05(24) ´ 105 y, by specific activity and mass spectrometric analysis [4]; 7.8 (5) ´ 105 y, by specific activity from a source produced by 26Mg(p,n) 26Al (the number of 26Al atoms in the source were estimated by using the reaction cross section) [5]; and 7.02 (56) ´ 105 y, by counting the atoms of 26Al that did not disintegrate in the source (Accelerator Mass Spectrometry) [6]. -

Hacking the Atom
Hacking the Atom EXPLORATIONS IN NUCLEAR RESEARCH, VOL. 1 The new science of low-energy nuclear reactions (1990-2015) Steven B. Krivit Edited by Michael J. Ravnitzky SAN RAFAEL, CALIFORNIA Hacking the Atom: Explorations in Nuclear Research, Vol. 1 Copyright © 2016 by Steven B. Krivit All rights reserved. No part of this book may be reproduced in whole or in part without written permission from the publisher, except by reviewers who may quote brief excerpts in connection with a review in a newspaper, magazine, or electronic publication; nor may any part of this book be reproduced, stored in a retrieval system, or transmitted in any form or by any means electronic without permission from the publisher. Pacific Oaks Press / New Energy Times / www.newenergytimes.com 369-B 3rd St. #556, San Rafael, CA 94901 Library of Congress Control Number: 2016902974 Krivit, Steven B., author. Hacking the atom / Steven B. Krivit ; editors, Michael Ravnitzky, Cynthia Goldstein, Mat Nieuwenhoven. pages cm -- (Explorations in nuclear research ; vol. 1) Includes bibliographical references and index. LCCN 2016902974 ISBN 978-0-996886444 (hbk.) ISBN 978-0-996886451 (pbk.) ISBN 978-0-996886468 (Kindle) ISBN 978-0-996886475 (ePUB) 1. Low-energy nuclear reactions--Research--History. 2. Electroweak interactions--Research--History. 3. Science--Social aspects. I. Ravnitzky, Michael, editor. II. Goldstein, Cynthia, editor. III. Nieuwenhoven, Mat, editor. IV. Title. V. Series: Krivit, Steven B. Explorations in nuclear research ; v. 1. QC794.8.L69K754 2016 539.7'5 QBI16-600060 Cover design: Lucien G. Frisch (Photograph: © Jahoo | Dreamstime.com) Interior design template: Book Design Templates Inc. Typeset in Crimson 11 pt., designed by Sebastian Kosch Editors: Michael Ravnitzky (Developmental Editor), Cynthia Goldstein (Copy Editor), Mat Nieuwenhoven (Technical Editor) Index: Laura Shelly Index Page numbers in bold indicate figures, Baldwin, Richard S., 52 tables, and photos. -

Radionuclides
Physics of Novel Radiation Modalities: Radionuclides James S. Welsh Stritch School of Medicine Loyola University Chicago Disclosure • Member of the Advisory Committee on the Medical Uses of Isotopes (ACMUI) for the United States Nuclear Regulatory Commission (NRC) • Board of directors: – Coqui Radioisotopes – Colossal Fossils Learning Objectives • Understand the basic physics of alpha, beta, gamma and other types of radioactivity • Gain some familiarity with the various sealed and unsealed radionuclides commonly used in radiation oncology Types of radioactivity • Alpha • Beta – Beta minus – beta plus (positron emission) – electron capture • Gamma – Isomeric transitions – Internal conversion – Internal pair production • Cluster radioactivity • Spontaneous fission – Binary or ternary • Rare types: – Proton radioactivity – b+ delayed proton emission – b- delayed neutron emission – b+ delayed deuteron or triton emission – Beta delayed fission Fun with Isotopes Radioactive decay supposedly follows a mathematically precise exponential function • Supposedly unaffected by temperature, pressure, chemical environment • First declared by Rutherford, Chadwick and Ellis Generally true but… …well-known exceptions do exist …well-known exceptions do exist • Electron Capture (e.g. 7Be, 109In, 110Sn) – If chemical environment make K-shell electrons less accessible, decay rate might be altered …well-known exceptions do exist • Electron Capture (e.g. 7Be, 109In, 110Sn) – If chemical environment make K-shell electrons less accessible, decay rate might be altered -

The Capture of Orbital Electrons by Nuclei Introduction
Luis W. Alvarez Phys. Rev. 54, 486 1938 The Capture of Orbital Electrons by Nuclei Luis W. Alvarez Radiation Laboratory, Physics Department, University of California, Berkeley, California (Received July 26, 1938) Abstract The simple theory of electron capture is outlined and three general methods for its detection are suggested. The first experimental evi- dence for the process (in activated titanium) is described. A rigorous experimental proof of the hypothesis ie given for the case of Ga67.A summary of several isotopes whose properties are best explained on this hypothesis is appended. The properties of Ga67 are described in considerable detail, and include the first evidence for internal conver- sion in artificially radioactive atoms. Introduction The suggestion that positron emitters might decay by the alternate process of electron capture was first advanced by Yukawa1 from considerations based on the Fermi theory of beta-ray emission. In this theory, the electrons and positrons are pictured as being created at the moment they are ejected, during neutron-proton transitions. The continuous beta-ray spectrum and the conservation of spin are explained by the simultaneous emission of a neutrino and electron. One may represent the transition involved in electron and positron decay by the following equations: N P + e− + ν (1) → P N + e+ + ν. (2) → 1Yukawa and Sakata, Proc. Phys. Math. Soc. Japan 17, 467 (1935); 18, 128 (1936). 1 On the basis of Dirac’s theory, however, the positron is merely the “hole” left in the continuum of negative energy electrons when one of these electrons is given a positive energy by the addition of at least 2mc2. -

Overlooked Potential of Positrons in Cancer Therapy Takanori Hioki1,3,4*, Yaser H
www.nature.com/scientificreports OPEN Overlooked potential of positrons in cancer therapy Takanori Hioki1,3,4*, Yaser H. Gholami1,3,4, Kelly J. McKelvey3,4, Alireza Aslani2,5, Harry Marquis1,4, Enid M. Eslick2, Kathy P. Willowson1,2, Viive M. Howell3,4,5 & Dale L. Bailey2,4,5* Positron (β+) emitting radionuclides have been used for positron emission tomography (PET) imaging in diagnostic medicine since its development in the 1950s. Development of a fuorinated glucose analog, fuorodeoxyglucose, labelled with a β+ emitter fuorine-18 (18F-FDG), made it possible to image cellular targets with high glycolytic metabolism. These targets include cancer cells based on increased aerobic metabolism due to the Warburg efect, and thus, 18F-FDG is a staple in nuclear medicine clinics globally. However, due to its attention in the diagnostic setting, the therapeutic potential of β+ emitters have been overlooked in cancer medicine. Here we show the frst in vitro evidence of β+ emitter cytotoxicity on prostate cancer cell line LNCaP C4-2B when treated with 20 Gy of 18F. Monte Carlo simulation revealed thermalized positrons (sub-keV) traversing DNA can be lethal due to highly localized energy deposition during the thermalization and annihilation processes. The computed single and double strand breakages were ~ 55% and 117% respectively, when compared to electrons at 400 eV. Our in vitro and in silico data imply an unexplored therapeutic potential for β+ emitters. These results may also have implications for emerging cancer theranostic strategies, where β+ emitting radionuclides could be utilized as a therapeutic as well as a diagnostic agent once the challenges in radiation safety and protection after patient administration of a radioactive compound are overcome. -

1 Radioactive Decay and the Origin Of
1 Radioactive Decay and the Origin of Gamma and X-Radiation 1.1 INTRODUCTION A, N and Z are all integers by definition. In practice, a neutron has a very similar mass to a proton and so there is In this chapter I intend to show how a basic understanding a real physical justification for this usage. In general, an of simple decay schemes, and of the role gamma radiation assembly of nucleons, with its associated electrons, should plays in these, can help in identifying radioactive nuclides be referred to as a nuclide. Conventionally, a nuclide of and in correctly measuring quantities of such nuclides. In atomic number Z, and mass number A is specified as ASy, doing so, I need to introduce some elementary concepts Z where Sy is the chemical symbol of the element. (This of nuclear stability and radioactive decay. X-radiation can format could be said to allow the physics to be defined be detected by using the same or similar equipment and I before the symbol and leave room for chemical informa- will also discuss the origin of X-rays in decay processes + tion to follow; for example, Co2 .) Thus, 58Co is a nuclide and the light that this knowledge sheds on characterization 27 with 27 protons and 31 neutrons. Because the chemical procedures. symbol uniquely identifies the element, unless there is a I will show how the Karlsruhe Chart of the Nuclides particular reason for including it, the atomic number as can be of help in predicting or confirming the identity of subscript is usually omitted – as in 58Co. -

Chapter 24 : Nuclear Reactions and Their Applications 24.1 Radioactive Decay and Nuclear Stability
Chapter 24 : Nuclear Reactions and Their Applications 24.1 Radioactive Decay and Nuclear Stability 24.2 The Kinetics of Radioactive Decay 24.3 Nuclear Transmutation: Induced Changes in Nuclei 24.4 The Effects of Nuclear Radiation on Matter 24.5 Applications of Radioisotopes 24.6 The Interconversion of Mass and Energy 24.7 Applications of Fission and Fusion Comparison of Chemical and Nuclear Reactions Chemical Reactions Nuclear Reactions 1. One substance is converted to 1. Atoms of one element typically another, but atoms never change change into atoms of another. identity. 2. Orbital electrons are involved as 2. Protons, neutrons, and other bonds break and form; nuclear particles are involved; orbital particles do not take part. electrons rarely take part. 3. Reactions are accompanied by 3.Reactions are accompanied by relatively small changes in energy relatively large changes in energy and no measurable changes in mass. and measurable changes in mass. 4. Reaction rates are influenced by 4. Reaction rates are affected by temperature, concentration, number of nuclei, but not by catalysts, and the compound in temperature, catalysts, or the which an element occurs. compound in which an element Table 24.1 occurs. A + 0 ZX A - mass number = p + n Z - number of protons(atomic no.) = p+ Number of neutrons(N) in a nucleus N = A - Z 238 0 92U N = 238 - 92 = 146 of n 238 - + 92U has 92e 92 p 235U - atomic bomb Subatomic elementary particles 0 Electron -1 e 1 Proton 1 p 1 Neutron 0n Nuclide - nuclear species with specific numbers of two types of -

Electron and Light Emission from Island Metal Films and Generation of Hot Electrons in Nanoparticles
R.D. Fedorovich et al. / Physics Reports 328 (2000) 73}179 73 ELECTRON AND LIGHT EMISSION FROM ISLAND METAL FILMS AND GENERATION OF HOT ELECTRONS IN NANOPARTICLES R.D. FEDOROVICH, A.G. NAUMOVETS, P.M. TOMCHUK Institute of Physics, National Academy of Sciences of Ukraine, 46 Prospect Nauki, UA-03039, Kiev 39, Ukraine AMSTERDAM } LAUSANNE } NEW YORK } OXFORD } SHANNON } TOKYO Physics Reports 328 (2000) 73}179 Electron and light emission from island metal "lms and generation of hot electrons in nanoparticles R.D. Fedorovich, A.G. Naumovets*, P.M. Tomchuk Institute of Physics, National Academy of Sciences of Ukraine, 46 Prospect Nauki, UA-03039, Kiev 39, Ukraine Received July 1999; editor: G. Comsa Contents 1. Introduction and outline 76 5. Hot electrons in metal nanoparticles 108 2. Island metal "lms: preparation and major 5.1. Introductory remarks 108 experimental "ndings 77 5.2. Heating up of electrons 109 2.1. Substrates and contact electrodes 77 5.3. A model of heating of the electron gas 2.2. Preparation of IMFs 78 in IMFs 112 2.3. Electroforming of IMFs 81 5.4. Phenomena caused by hot electrons in 2.4. Emission centers 83 IMFs 114 2.5. Chain island "lms 85 5.5. Mechanisms of light emission from 3. Electrical conductivity and electron emission IMFs 115 properties of IMFs 86 5.6. Summary of Section 5 121 3.1. Major experimental "ndings in brief 86 6. Electron}lattice energy exchange in small metal 3.2. Electrical conductivity of IMFs 87 particles 122 3.3. Electron emission from IMFs under 6.1. -

Baggott J. the Quantum Story (OUP, 2011)(ISBN 0199566844)(O
the quantum story This page intentionally left blank THE Quantum Story a history in 40 moments jim baggott 1 1 Great Clarendon Street, Oxford ox2 6dp Oxford University Press is a department of the University of Oxford. It furthers the University’s objective of excellence in research, scholarship, and education by publishing worldwide in Oxford New York Auckland Cape Town Dar es Salaam Hong Kong Karachi Kuala Lumpur Madrid Melbourne Mexico City Nairobi New Delhi Shanghai Taipei Toronto With offi ces in Argentina Austria Brazil Chile Czech Republic France Greece Guatemala Hungary Italy Japan Poland Portugal Singapore South Korea Switzerland Thailand Turkey Ukraine Vietnam Oxford is a registered trade mark of Oxford University Press in the UK and in certain other countries Published in the United States by Oxford University Press Inc., New York © Jim Baggott 2011 The moral rights of the authors have been asserted Database right Oxford University Press (maker) First published 2011 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of Oxford University Press, or as expressly permitted by law, or under terms agreed with the appropriate reprographics rights organization. Enquiries concerning reproduction outside the scope of the above should be sent to the Rights Department, Oxford University Press, at the address above You must not circulate this book in any other binding or cover and you must impose the same condition -

Library of Recommended Actinide Decay Data, 2011
Library of Recommended Actinide Decay Data, 2011 Actinide Decay Data, Library of Recommended Library of Recommended Actinide Decay Data, 2011 High quality decay data are an essential input across a wide range of nuclear applications, and none more so than in the case of the actinides and their related decay chain data. Well defi ned nuclear data are essential to ensure safe procedures within mining operations, various nuclear fuel cycles for energy generation, environmental monitoring, specifi c analytical techniques, and diagnostic and radiotherapeutic treatments in nuclear medicine. A major objective of the IAEA nuclear data programme is to promote improvements in the accuracy and quality of nuclear data used in science and technology. The contents of this report constitute the results of a coordinated research project established to assemble an updated decay data library for actinides. Recommended half-lives and decay scheme data have been comprehensively evaluated, and are tabulated in terms of a carefully selected set of actinide radionuclides. Technical Editors M.A. Kellett, A.L. Nichols INTERNATIONAL ATOMIC ENERGY AGENCY VIENNA ISBN 978 –92–0–143910–9 1 LIBRARY OF RECOMMENDED ACTINIDE DECAY DATA, 2011 The following States are Members of the International Atomic Energy Agency: AFGHANISTAN GUATEMALA PANAMA ALBANIA HAITI PAPUA NEW GUINEA ALGERIA HOLY SEE PARAGUAY ANGOLA HONDURAS PERU ARGENTINA HUNGARY PHILIPPINES ARMENIA ICELAND POLAND AUSTRALIA INDIA PORTUGAL AUSTRIA INDONESIA QATAR AZERBAIJAN IRAN, ISLAMIC REPUBLIC OF BAHRAIN IRAQ