Oxidation-Reduction Alternating Copolymerization of Germylene and N-Phenyl-P-Quinoneimine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cosmogenic Production As a Background in Searching For
Cosmogenic Production as a Background in Searching for Rare Physics Processes D.-M. Mei a , Z.-B. Yin a,b,c,1, S. R. Elliott d aDepartment of Physics, The University of South Dakota, Vermillion, South Dakota 57069 bInstitute of Particle Physics, Huazhong Normal University, Wuhan 430079, China cKey Laboratory of Quark & Lepton Physics (Huazhong Normal University), Ministry of Education, China dLos Alamos National Laboratory, Los Alamos, New Mexico 87545 Abstract We revisit calculations of the cosmogenic production rates for several long-lived iso- topes that are potential sources of background in searching for rare physics processes such as the detection of dark matter and neutrinoless double-beta decay. Using up- dated cosmic-ray neutron flux measurements, we use TALYS 1.0 to investigate the cosmogenic activation of stable isotopes of several detector targets and find that the cosmogenic isotopes produced inside the target materials and cryostat can result in large backgrounds for dark matter searches and neutrinoless double-beta decay. We use previously published low-background HPGe data to constrain the production of 3H on the surface and the upper limit is consistent with our calculation. We note that cosmogenic production of several isotopes in various targets can generate po- arXiv:0903.2273v1 [nucl-ex] 12 Mar 2009 tential backgrounds for dark matter detection and neutrinoless double-beta decay with a massive detector, thus great care should be taken to limit and/or deal with the cosmogenic activation of the targets. Key words: Cosmogenic activation, Dark matter detection, Double-beta decay PACS: 13.85.Tp, 23.40-s, 25.40.Sc, 28.41.Qb, 95.35.+d, 29.40.Wk Email address: [email protected] (D.-M. -

Direct Measurement of the Neutron
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 1-9-2020 Stellar Nucleosynthesis: Direct Measurement of the Neutron- Capture Cross Sections of Stable Germanium Isotopes and Design of a Next Generation Ion Trap for the Study of Beta- Delayed Neutron Emission Alexander Laminack Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Instrumentation Commons, Nuclear Commons, Physical Processes Commons, and the Stars, Interstellar Medium and the Galaxy Commons Recommended Citation Laminack, Alexander, "Stellar Nucleosynthesis: Direct Measurement of the Neutron-Capture Cross Sections of Stable Germanium Isotopes and Design of a Next Generation Ion Trap for the Study of Beta- Delayed Neutron Emission" (2020). LSU Doctoral Dissertations. 5131. https://digitalcommons.lsu.edu/gradschool_dissertations/5131 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. STELLAR NUCLEOSYNTHESIS: DIRECT MEASUREMENT OF THE NEUTRON-CAPTURE CROSS SECTIONS OF STABLE GERMANIUM ISOTOPES AND DESIGN OF A NEXT GENERATION ION TRAP FOR THE STUDY OF β-DELAYED NEUTRON EMISSION A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Physics and Astronomy by Alexander Laminack B. S., The Unviersity of Alabama, 2015 May 2020 To my wife and son: Kristy Allen Alexander Laminack and Daniel Allen Laminack. -

The New Nuclear Forensics: Analysis of Nuclear Material for Security
THE NEW NUCLEAR FORENSICS Analysis of Nuclear Materials for Security Purposes edited by vitaly fedchenko The New Nuclear Forensics Analysis of Nuclear Materials for Security Purposes STOCKHOLM INTERNATIONAL PEACE RESEARCH INSTITUTE SIPRI is an independent international institute dedicated to research into conflict, armaments, arms control and disarmament. Established in 1966, SIPRI provides data, analysis and recommendations, based on open sources, to policymakers, researchers, media and the interested public. The Governing Board is not responsible for the views expressed in the publications of the Institute. GOVERNING BOARD Sven-Olof Petersson, Chairman (Sweden) Dr Dewi Fortuna Anwar (Indonesia) Dr Vladimir Baranovsky (Russia) Ambassador Lakhdar Brahimi (Algeria) Jayantha Dhanapala (Sri Lanka) Ambassador Wolfgang Ischinger (Germany) Professor Mary Kaldor (United Kingdom) The Director DIRECTOR Dr Ian Anthony (United Kingdom) Signalistgatan 9 SE-169 70 Solna, Sweden Telephone: +46 8 655 97 00 Fax: +46 8 655 97 33 Email: [email protected] Internet: www.sipri.org The New Nuclear Forensics Analysis of Nuclear Materials for Security Purposes EDITED BY VITALY FEDCHENKO OXFORD UNIVERSITY PRESS 2015 1 Great Clarendon Street, Oxford OX2 6DP, United Kingdom Oxford University Press is a department of the University of Oxford. It furthers the University’s objective of excellence in research, scholarship, and education by publishing worldwide. Oxford is a registered trade mark of Oxford University Press in the UK and in certain other countries © SIPRI 2015 The moral rights of the authors have been asserted All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of SIPRI, or as expressly permitted by law, or under terms agreed with the appropriate reprographics rights organizations. -

Activation Cross Sections of Longer-Lived Radionuclides Produced in Germanium by Alpha Particle Irradiation
Title Activation cross sections of longer-lived radionuclides produced in germanium by alpha particle irradiation Author(s) Takacs, S.; Takacs, M. P.; Ditroi, F.; Aikawa, M.; Raba, H.; Komori, Y. Nuclear Instruments and Methods in Physics Research Section B : Beam Interactions with Materials and Atoms, 383, Citation 213-226 https://doi.org/10.1016/j.nimb.2016.07.015 Issue Date 2016-09-15 Doc URL http://hdl.handle.net/2115/71220 c2016, Elsevier. Licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Rights http://creativecommons.org/licenses/by-nc-nd/4.0/ Rights(URL) http://creativecommons.org/licenses/by-nc-nd/4.0/ Type article (author version) File Information NIMPR B383 213-226.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Activation cross sections of longer-lived radionuclides produced in germanium by alpha particle irradiation S. Takács1,*, M.P. Takács1,†, F. Ditrói1, M. Aikawa2, H. Haba3, Y. Komori3 1 Institute for Nuclear Research, Hungarian Academy of Sciences, Atomki, 4026 Debrecen, Hungary 2 Faculty of Science, Hokkaido University, Sapporo 060-0810, Japan 3 Nishina Center for Accelerator-Based Science, RIKEN, Wako, Saitama 351-0198, Japan Abstract The cross sections of alpha particles induced nuclear reactions on natural germanium were investigated by using the standard stacked foil target technique, the activation method and high resolution gamma spectrometry. Targets with thickness of about 1 μm were prepared from natural Ge by vacuum evaporation onto 25 μm thick polyimide (Kapton) backing foils. Stacks were composed of Kapton-Ge-Ge-Kapton sandwich target foils and additional titanium monitor foils with nominal thickness of 11 micrometers to monitor the beam parameters using nat 51 the Ti(,x) Cr reaction. -

Nuclear Mass and Stability
CHAPTER 3 Nuclear Mass and Stability Contents 3.1. Patterns of nuclear stability 41 3.2. Neutron to proton ratio 43 3.3. Mass defect 45 3.4. Binding energy 47 3.5. Nuclear radius 48 3.6. Semiempirical mass equation 50 3.7. Valley of $-stability 51 3.8. The missing elements: 43Tc and 61Pm 53 3.8.1. Promethium 53 3.8.2. Technetium 54 3.9. Other modes of instability 56 3.10. Exercises 56 3.11. Literature 57 3.1. Patterns of nuclear stability There are approximately 275 different nuclei which have shown no evidence of radioactive decay and, hence, are said to be stable with respect to radioactive decay. When these nuclei are compared for their constituent nucleons, we find that approximately 60% of them have both an even number of protons and an even number of neutrons (even-even nuclei). The remaining 40% are about equally divided between those that have an even number of protons and an odd number of neutrons (even-odd nuclei) and those with an odd number of protons and an even number of neutrons (odd-even nuclei). There are only 5 stable nuclei known which have both 2 6 10 14 an odd number of protons and odd number of neutrons (odd-odd nuclei); 1H, 3Li, 5B, 7N, and 50 23V. It is significant that the first stable odd-odd nuclei are abundant in the very light elements 2 (the low abundance of 1H has a special explanation, see Ch. 17). The last nuclide is found in low isotopic abundance (0.25%) and we cannot be certain that this nuclide is not unstable to radioactive decay with extremely long half-life. -

(N,{Alpha}) Cross Sections in the 14 Mev Region
XA9744740 International Atomic Energy Agency INDC(HUN)-031 Distr. L I N DC INTERNATIONAL NUCLEAR DATA COMMITTEE INVESTIGATIONS ON (n,a) CROSS SECTIONS IN THE 14 MeV REGION A.D. Majdeddin 1, V. Semkova 2, R. Doczi3, Cs.M. Buczko 3 and J. Csikai3 'Faculty of Nuclear and Electronic Engineering, Al-Fateh University, P.O. Box 13292 Tripoli, Libya institute of Nuclear Physics and Nuclear Energy, Bulgarian Academy of Sciences Tzarigradsko 72, 1784 Sofia, Bulgaria institute of Experimental Physics, Kossuth University, 4001 Debrecen, Pf. 105, Hungary July 1997 IAEA NUCLEAR DATA SECTION, WAGRAMERSTRASSE 5, A-1400 VIENNA Reproduced by the IAEA in Austria July 1997 INDC(HUN)-031 Distr. L INVESTIGATIONS ON (n,a) CROSS SECTIONS IN THE 14 MeV REGION A.D. Majdeddin 1, V. Semkova 2, R. Doczi3, Cs.M. Buczko 3 and J. Csikai3 'Faculty of Nuclear and Electronic Engineering, Al-Fateh University, P.O. Box 13292 Tripoli, Libya institute of Nuclear Physics and Nuclear Energy, Bulgarian Academy of Sciences Tzarigradsko 72, 1784 Sofia, Bulgaria institute ofExperimental Physics, Kossuth University, 4001 Debrecen, Pf. 105, Hungary July 1997 INVESTIGATIONS ON (n,a) CROSS SECTIONS IN THE 14 MeV REGION A.D.Majdeddin 1, V.Semkova 2, R.Doczi3, Cs.M.Buczkd 3 and J.Csikai3 'Faculty of Nuclear and Electronic Engineering, Al-Fateh University, P.O.Boxl3292 Tripoli,Libya ^ Institute of Nuclear Physics and Nuclear Energy, Bulgarian Academy of Sciences Tzarigradsko 72,1784 Sofia,Bulgaria ^Institute of Experimental Physics, Kossuth University, 4001 Debrecen, Pf.105, Hungary Cross sections have been measured, deduced and adopted for 183 (n,a) reactions at (14.7+0.1) MeV incident neutron energy. -

Neutron Capture Cross Sections of 70,72,73,74,76Ge at N TOF EAR-1
EUROPEAN ORGANIZATION FOR NUCLEAR RESEARCH Proposal to the ISOLDE and Neutron Time-of-Flight Committee Neutron capture cross sections of 70;72;73;74;76Ge at n TOF EAR-1 September 23, 2013 C. Lederer1, J. Andrzejewski2, M. Barbagallo3, E. Chiaveri4, C. Domingo-Pardo5, R. Dressler6, P. Ferreira7, I.F. Gon¸calves7, C. Guerrero4, F. Gunsing8, F. K¨appeler9, J. Neuhausen6, C. Massimi10, J. Perkowski2, R. Reifarth1, D. Schumann6, G. Tagliente3, J.L. Tain5, P. Vaz7, A. Wallner11, C. Weiß4;12, and the n TOF Collaboration. 1Goethe University Frankfurt, Germany 2UniwersytetL´odzki,Lodz, Poland 3INFN Bari, Italy 4CERN, Switzerland 5IFIC Valencia, Spain 6PSI, Switzerland 7Universidade T´ecnica de Lisboa, Lisboa, Portugal 8CEA Saclay, France 9Karlsruhe Institute of Technology, Germany 10INFN and University of Bologna, Italy 11Australian National University, Australia 12Technical University of Vienna, Austria Spokesperson: C. Lederer, [email protected] Technical coordinator: O. Aberle, [email protected] Abstract: We propose to measure the (n; γ) cross sections of the isotopes CERN-INTC-2013-021 / INTC-P-381 23/09/2013 70;72;73;74;76Ge. Neutron induced reactions on Ge are of importance for the astrophysical slow neutron capture process, which is responsible for forming about half of the overall elemental abundances heavier than Fe. The neutron capture cross section on Ge affects the abundances produced in this process for a number of heavier isotopes up to a mass number of A = 90. Additionally, neutron capture on Ge is of interest for low background experiments involving Ge detectors. Experimental cross section data presently available for Ge(n; γ) are scarce and cover only a fraction of the neutron energy range of interest. -

Accelerator-Based Production of High Specific Activity Radionuclides for Radiopharmaceutical Applications
ACCELERATOR-BASED PRODUCTION OF HIGH SPECIFIC ACTIVITY RADIONUCLIDES FOR RADIOPHARMACEUTICAL APPLICATIONS A Dissertation Presented to the Faculty of the Graduate School at the University of Missouri-Columbia In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy By MATTHEW DAVID GOTT Dr. Silvia S. Jurisson, Dissertation Advisor Dr. Cathy S. Cutler, Co-Advisor JULY 2015 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled ACCELERATOR-BASED PRODUCTION OF HIGH SPECIFIC ACTIVITY RADIONUCLIDES FOR RADIOPHARMACEUTICAL APPLICATIONS presented by Matthew David Gott, a candidate for the degree of Doctor of Philosophy, and hereby certify that, in their opinion, it is worthy of acceptance. Dr. Silvia S. Jurisson Dr. Cathy S. Cutler Dr. C. Michael Greenlief Dr. J. David Robertson DEDICATION This dissertation is dedicated to my wonderful parents, David and Cathy. You have always been my greatest supporters and encouraged me every step of the way. None of this would have been possible without you. ACKNOWLEDGMENTS I would like to thank Dr. C. Michael Greenlief and Dr. J. David Roberston for serving on my committee and providing advice throughout this process. I would like to thank all of my collaborators who provided assistance and guidance throughout this project. Dr. Donald Wycoff, Dr. Anthony Degraffenreid, and Yutian Feng were instrumental in the arsenic production work. Dr. Alan Ketring, Dr. John Lydon, Mary Embree, Stacy Wilder, Alex Saale, Melissa Evans-Blumer, and the staff at the University of Missouri Research Reactor provided support and ideas for experimental work throughout this dissertation. Dr. Michael Fassbender, Dr. Beau Ballard, and the members of the C-IIAC group at Los Alamos National Laboratory provided assistance and guidance with the experimental work for the W/Re separation method. -

Graduate Students Receiving Ph.D. Degrees in Physics and Astronomy
Appendix Three Graduate Students Receiving Ph.D. Degrees in Physics and Astronomy Year Recipient Thesis Dissertation Advisor 1935 Hill, Donald M. Winchester “The Principal Expansion Coefficient of Single Crystals of Mercury” 1936 Downsbrough, G. Atkinson “The Damping of Torsional Oscillations in Quartz Fibers” 1939 Buc, George L. Winchester/ “Ultraviolet Absorption Spectra of Three Greenlees Related Olefines” 1949 Hsiang, Jen-Sen Whitmer “Theoretical Studies of Ferromagnetics” 1950 Hemenway, Curtis Dunnington “A Determination of the Ratio of Planck's Constant to the Charge of an Electron” 1950 Thomas, Jay T. Torrey “A Study of the Solid State by Means of the Nuclear Magnetic Resonance Pulse Technique” 1951 Baker, Paul E. Whitmer “A Study of the Dielectric Properties of Single Crystal Alums as a Function of Temperature at 9450 Megacycles per Second” 1951 Garfunkel, Myron Serin “A Study of the Formation of a Boundary Between Normal-Conducting and Super-Conducting Metal” 1951 Ginsburg, Irvin Torrey “Nuclear Resonance Studies of Hydrogen - Palladium Alloys” 1951 Gray, Sidney Torrey “A Study of the Long Pulse Method for Investigating Nuclear Magnetic Resonance Relaxation Times” 1952 Eschenfelder, A. Weidner “Saturation Effects in Paramagnetic Resonance Absorption” 1952 Gittleman, J. Serin “A Study of the Transition from the Superconducting State to the Normal 232 Physics and Astronomy Ph.D. Degrees State of a Hollow Tin Cylinder in a Uniform Magnetic Field” 1952 Wright, Wilbur H. Serin “A Study of Phase Transition Phenomena in Superconductors” -

Foia/Pa-2015-0050
Acknowledgements The following were the members of ICRP Committee 2 who prepared this report. J. Vennart (Chairman); W. J. Bair; L. E. Feinendegen; Mary R. Ford; A. Kaul; C. W. Mays; J. C. Nenot; B. No∈ P. V. Ramzaev; C. R. Richmond; R. C. Thompson and N. Veall. The committee wishes to record its appreciation of the substantial amount of work undertaken by N. Adams and M. C. Thorne in the collection of data and preparation of this report, and, also, to thank P. E. Morrow, a former member of the committee, for his review of the data on inhaled radionuclides, and Joan Rowley for secretarial assistance, and invaluable help with the management of the data. The dosimetric calculations were undertaken by a task group, centred at the Oak Ridge Nntinnal-..._._.__. ---...“..,-J,1 .nhnmtnrv __ac ..,.I_.,“.fnllnwr~ Mary R. Ford (Chairwoman), S. R. Bernard, L. T. Dillman, K. F. Eckerman and Sarah B. Watson. Committee 2 wishes to record its indebtedness to the task group for the completion of this exacting task. The data given in this report are to be used together with the text and dosimetric models described in Part 1 of ICRP Publication 30;’ the chapters referred to in this preface relate to that report. In order to derive values of the Annual Limit on Intake (ALI) for radioisotopes of scandium, the following assumptions have been made. In the metabolic data for scandium, a fraction 0.4 of the element in the transfer compartment is translocated to the skeleton. It is assumed thit this scandium in the skeleton is distributed between cortical bone, trabecular bone and red marrow in proportion to their respective masses. -

On the Underground Production of High Purity Germanium Detectors
On the Underground Production of High Purity Germanium Detectors 1 2 3 3 Mikael Hult , Sergey Belogurov , Allan Caldwell , Josef Janicsko , 2,4 5 Vasiliy Kornoukhov , Stefan Schönert 1 European Commission-Joint Research Centre-Institute for Reference Materials and Measurements (EC-JRC-IRMM), Geel, Belgium 2 Institute for Nuclear Research of the Russian Academy of Sciences, Moscow, Russia 3 Max Planck Institut für Physik, München, Germany 4 Institute of Theoretical and Experimental Physics, Moscow, Russia 5 Max Planck Institut für Kernphysik, Heidelberg, Germany EUR 23237 EN - 2008 The mission of the IRMM is to promote a common and reliable European measurement system in support of EU policies. European Commission Joint Research Centre Institute for Reference Materials and Measurements Contact information Mikael Hult Institute for Reference Materials and Measurements Retieseweg 111 B-2440 Geel, Belgium E-mail: [email protected] Tel.: +32-(0)14-571 269 Fax: +32-(0)14-571 864 http://irmm.jrc.ec.europa.eu/ http://www.jrc.ec.europa.eu/ Legal Notice Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of this publication. Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed. A great deal of additional information on the European Union is available on the Internet. It can -
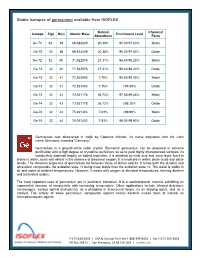
Stable Isotopes of Germanium Available from ISOFLEX
Stable isotopes of germanium available from ISOFLEX Natural Chemical Isotope Z(p) N(n) Atomic Mass Enrichment Level Abundance Form Ge-70 32 38 69.924249 20.38% 95.30-97.60% Metal Ge-70 32 38 69.924249 20.38% 95.30-97.60% Oxide Ge-72 32 40 71.922076 27.31% 96.40-98.20% Metal Ge-72 32 40 71.922076 27.31% 96.40-98.20% Oxide Ge-73 32 41 72.923460 7.76% 95.60-99.40% Metal Ge-73 32 41 72.923460 7.76% >95.50% Oxide Ge-74 32 42 73.921178 36.72% 97.50-99.80% Metal Ge-74 32 42 73.921178 36.72% ≥95.20% Oxide Ge-76 32 44 75.921403 7.83% >99.90% Metal Ge-76 32 44 75.921403 7.83% 88.00-99.90% Oxide Germanium was discovered in 1886 by Clemens Winkler. Its name originates with the Latin name Germania, meaning "Germany." Germanium is a grayish-white cubic crystal. Elemental germanium can be prepared in extreme purification with a high degree of crystalline perfection, so as to yield highly-characterized surfaces. Its conductivity depends largely on added impurities. It is attacked by nitric acid and aqua regia, but it is stable in water, acids and alkalis in the absence of dissolved oxygen. It is insoluble in water, dilute acids and dilute alkalis. The chemical properties of germanium fall between those of silicon and tin. It forms both the divalent and tetravalent compounds, the oxidation state +4 being more stable than the oxidation state +2.