(12) Patent Application Publication (10) Pub. No.: US 2004/0234450 A1 Howes (43) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spectroscopy of Neutron-Rich 34,35,36,37,38 P Populated In
PHYSICAL REVIEW C 92, 044308 (2015) , , , , Spectroscopy of neutron-rich 34 35 36 37 38P populated in binary grazing reactions R. Chapman,1,* A. Hodsdon,1 M. Bouhelal,2 F. Haas,3 X. Liang,1 F. Azaiez,4 Z. M. Wang,1 B. R. Behera,5 M. Burns,1 E. Caurier,3 L. Corradi,5 D. Curien,3 A. N. Deacon,6 Zs. Dombradi,´ 7 E. Farnea,8 E. Fioretto,5 A. Gadea,5 F. Ibrahim,4 A. Jungclaus,9 K. Keyes,1 V. Kumar, 1 S. Lunardi,8 N. Marginean,˘ 5,10 G. Montagnoli,8 D. R. Napoli,5 F. Nowacki,3 J. Ollier,1,11 D. O’Donnell,1,12 A. Papenberg,1 G. Pollarolo,13 M.-D. Salsac,14 F. Scarlassara,8 J. F. Smith,1 K. M. Spohr,1 M. Stanoiu,10 A. M. Stefanini,5 S. Szilner,5,15 M. Trotta,5 and D. Verney4 1School of Engineering and Computing, University of the West of Scotland, Paisley PA1 2BE, United Kingdom, and The Scottish Universities Physics Alliance (SUPA) 2Laboratoire de Physique Appliquee´ et Theorique,´ Universite´ Larbi Tebessa,´ Tebessa,´ Algerie´ 3IPHC, UMR7178, CNRS-IN2P3, and Universite´ de Strasbourg, F-67037 Strasbourg Cedex 2, France 4IPN, IN2P3-CNRS and Universite´ Paris-Sud, F-91406 Orsay Cedex, France 5INFN, Laboratori Nazionali di Legnaro, I-35020 Legnaro, Padova, Italy 6Schuster Laboratory, University of Manchester, Manchester M13 9PL, United Kingdom 7MTA ATOMKI, P.O. Box 51, H-4001 Debrecen, Hungary 8Dipartimento di Fisica and INFN-Sezione di Padova, Universita` di Padova, I35131 Padova, Italy 9Instituto de Estructura de la Materia, CSIC, E-28006 Madrid, Spain 10Horia Hulubei National Institute of Physics and Nuclear Engineering-IFIN-HH, Strasse Atomistilor No. -

Stable Isotopes of Cobalt Properties of Cobalt
Stable Isotopes of Cobalt Isotope Z(p) N(n) Atomic Mass Natural Abundance Nuclear Spin Co-59 27 32 58.93320 100.00% 7/2- Cobalt was discovered in 1735 by Georg Brandt. Its name derives from the German word kobald, meaning "goblin" or "evil spirit." Minerals containing cobalt were used by the early civilizations of Egypt and Mesopotamia for coloring glass deep blue. Cobalt oxide is used today to add a pink or blue color to glass. It is also an important trace element in soils and necessary for animal nutrition. The most important modern use of cobalt is in the manufacture of various wear-resistant and superalloys. Its alloys have shown high resistance to corrosion and oxidation at high temperatures. Radioactive Cobalt-60 is used in radiography and in the sterilization of food. A silvery-white, shining, hard, ductile, somewhat malleable metal, cobalt is also ferromagnetic, with permeability two-thirds that of iron. It has exceptional magnetic properties in alloys. It is attached by dilute hydrochloric and sulfuric acids. It corrodes readily in air, and it has unusual coordinating properties, especially the trivalent ion. It is noncombustible except in powder form. Cobalt occurs in two allotropic modifications over a wide range of temperatures: the crystalline close-packed- hexagonal form is known as the alpha form, which turns into the beta (or gamma) form above 417 ºC. In finely powdered form, cobalt ignites spontaneously in air. Reactions with acetylene and bromine pentafluoride proceed to incandescence and can become violent. The metal is moderately toxic by ingestion. Inhalation of dusts can damage lungs. -

Laser Spectroscopy of Neutron Rich Bismuth Isotopes
CERN LIBRARIES, GENEVA new lllllllllllIllllllllllllllllllllllllllllllll SC00000267 CD [L] Q D K,Q/pm lO CERN/ISC 94-7 P60 scP GEM} »- emit 1=·1>.o1=·osAL TO THE rsotos COMMITTEE 9 Le-? Laser Spectroscopy of Neutron Rich Bismuth Isotopes CERN1 — Mainz? — Manchester3 — Stony Brook4 Collaboration J. Behr4, J. Billowes3, P. Campbell3, T.G. Cooperg, U. Georg2, I.S. Grant3, G. Gwinner4, G. Huber], M. Keimz, J. Kilgallon3, A. Kleinz, R. Neugart2, M. Neurothz, G.D. Sprouse‘*, P.D. Wheelera, F. Xu‘* and the ISOLDE Collaboration] Spokesman: J. Billowes Contactman: G. Huber Summary The aim of the proposed experiment is to measure the optical isotope shifts and hyperfine structures of bismuth isotopes in the region 21°'2“Bi which lie above the N=126 shell closure. The change in nuclear mean square charge radii and static moments can be deduced. These will be the first isotones of lead to be measured immediately above the shell closure. This will provide new information on the systematics of the kink in the charge radii change with neutron number seen in the Pb isotopic chain, and will represent the first measurement of the odd proton hg/2 moment in the region above the closed 2°8Pb core. Laser resonance fluorescence will be used on the isotopic bismuth samples as they are released into a gas cell trap. This is a method that has been applied very successfully at SUNY, Stony Brook to the neutron deficient bismuth isotopes and it makes efficient use of the small samples available. The bismuth samples of 212Bi and 213Bi will be prepared by catching intense beams of 22°Fr and 2"Fr on thin lead foils. -

The Cyclotron Production and Cyclometalation Chemistry of 192-Ir
The Cyclotron Production and Cyclometalation Chemistry of 192-Ir by Graeme Langille B.Sc., Simon Fraser University, 2012 Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Department of Chemistry Faculty of Science c Graeme Langille 2014 SIMON FRASER UNIVERSITY Fall 2014 All rights reserved. However, in accordance with the Copyright Act of Canada, this work may be reproduced without authorization under the conditions for "Fair Dealing". Therefore, limited reproduction of this work for the purposes of private study, research, criticism, review and news reporting is likely to be in accordance with the law, particularly if cited appropriately. APPROVAL Name: Graeme Langille Degree: Master of Science (Chemistry) Title: The Cyclotron Production and Cyclometalation Chemistry of 192-Ir Examining Committee: Chair: Dr. Hua-Zhong Yu Professor Dr. Corina Andreoiu Senior Supervisor Associate Professor Dr. Paul Schaffer Co-Supervisor Adjunct Professor Dr. Tim Storr Supervisor Associate Professor Dr. Krzysztof Starosta Supervisor Associate Professor Dr. Robert Young Internal Examiner Professor Date Defended/Approved: December 11, 2014 ii Partial Copyright Licence iii Abstract The goal of this thesis is to demonstrate the cyclotron production, radiochemical isola- tion, and cyclometalate chemistry of radio-iridium isotopes. In recent work, Luminescence Cell Imaging (LCI) has been combined with radioisotopes, leading to compounds that can be imaged with both optical microscopy and nuclear techniques. Radiometals excel in this multifunctional setting, providing ideal chemical and nuclear properties for luminescence, bi- ological targeting, nuclear diagnostics, and therapy. Iridium cyclometalate compounds have demonstrated potential in LCI with excellent photophysical properties. Independently, low specific activity 192Ir has been successfully applied in brachytherapy as a high-intensity β− emitter. -

Circular of the Bureau of Standards No. 562: Bibliography of Research on Deuterium and Tritium Compounds 1945 and 1952
NBS CIRCULAR 562 Bibliography of Research on Deuterium and Tritium Compounds 1945 to 1952 UNITED STATES DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS PERIODICALS OF THE NATIONAL BUREAU OF STANDARDS (Published monthly) The National Bureau of Standards is engaged in fundamental and applied research in physics, chemistry, mathematics, and engineering. Projects are conducted in fifteen fields: electricity and electronics, optics and metrology, heat and power, atomic and radiation physics, chemistry, mechanics, organic and fibrous materials, metallurgy, mineral products, building technology, applied mathematics, data process¬ ing systems, cryogenic engineering, radio propagation, and radio standards. The Bureau has custody of the national standards of measurement and conducts research leading to the improvement of scientific and engineering standards and of techniques and methods of measurement. Testing methods and in¬ struments are developed; physical constants and properties of materials are determined; and technical processes are investigated. Journal of Research The Journal presents research papers by authorities in the specialized fields of physics, mathematics, chemistry, and engineering. Complete details of the work are presented, including laboratory data, experimental procedures, and theoretical and mathematical analyses. Annual subscription: domestic, $4.00; 25 cents additional for foreign mailing. Technical News Bulletin Summaries of current research at the National Bureau of Standards are published each month in the Technical News Bulletin. The articles are brief, with emphasis on the results of research, chosen on the basis of their scientific or technologic importance. Lists of all Bureau publications during the preceding month are given, including Research Papers, Handbooks, Applied Mathematics Series, Building Mate¬ rials and Structures Reports, Miscellaneous Publications, and Circulars. -

THE NATURAL RADIOACTIVITY of the BIOSPHERE (Prirodnaya Radioaktivnost' Iosfery)
XA04N2887 INIS-XA-N--259 L.A. Pertsov TRANSLATED FROM RUSSIAN Published for the U.S. Atomic Energy Commission and the National Science Foundation, Washington, D.C. by the Israel Program for Scientific Translations L. A. PERTSOV THE NATURAL RADIOACTIVITY OF THE BIOSPHERE (Prirodnaya Radioaktivnost' iosfery) Atomizdat NMoskva 1964 Translated from Russian Israel Program for Scientific Translations Jerusalem 1967 18 02 AEC-tr- 6714 Published Pursuant to an Agreement with THE U. S. ATOMIC ENERGY COMMISSION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D. C. Copyright (D 1967 Israel Program for scientific Translations Ltd. IPST Cat. No. 1802 Translated and Edited by IPST Staff Printed in Jerusalem by S. Monison Available from the U.S. DEPARTMENT OF COMMERCE Clearinghouse for Federal Scientific and Technical Information Springfield, Va. 22151 VI/ Table of Contents Introduction .1..................... Bibliography ...................................... 5 Chapter 1. GENESIS OF THE NATURAL RADIOACTIVITY OF THE BIOSPHERE ......................... 6 § Some historical problems...................... 6 § 2. Formation of natural radioactive isotopes of the earth ..... 7 §3. Radioactive isotope creation by cosmic radiation. ....... 11 §4. Distribution of radioactive isotopes in the earth ........ 12 § 5. The spread of radioactive isotopes over the earth's surface. ................................. 16 § 6. The cycle of natural radioactive isotopes in the biosphere. ................................ 18 Bibliography ................ .................. 22 Chapter 2. PHYSICAL AND BIOCHEMICAL PROPERTIES OF NATURAL RADIOACTIVE ISOTOPES. ........... 24 § 1. The contribution of individual radioactive isotopes to the total radioactivity of the biosphere. ............... 24 § 2. Properties of radioactive isotopes not belonging to radio- active families . ............ I............ 27 § 3. Properties of radioactive isotopes of the radioactive families. ................................ 38 § 4. Properties of radioactive isotopes of rare-earth elements . -

Annual Report 1951 National Bureau of Standards
Annual Report 1951 National Bureau of Standards Miscellaneous Publication 204 UNITED STATES DEPARTMENT OF COMMERCE Charles Sawyer, Secretary NATIONAL BUREAU OF STANDARDS A. V. Astint, Director Annual Report 1951 National Bureau of Standards For sale by the Superintendent of Documents, U. S. Government Printing Office Washington 2 5, D. C. Price 50 cents CONTENTS Page 1. General Review 1 2. Electricity 16 Beam intensification in a high-voltage oscillograph 17, Low-temperature dry cells 17, High-rate batteries 17, Battery additives 18. 3. Optics and Metrology 18 The kinorama 19, Measurement of visibility for aircraft 20, Antisubmarine aircraft searchlights 20, Resolving power chart 20, Refractivity 21, Thermal expansivity of aluminum alloys 21. 4. Heat and Power . 21 Thermodynamic properties of materials 22, Synthetic rubber and other high polymers 23, Combustors for jet engines 24, Temperature and composition of flames 25, Engine "knock" 25, Low-temperature physics 26, Medical physics instrumentation 28. 5. Atomic and Radiation Physics 28 Atomic standard of length 29, Magnetic moment of the proton 29, Spectra of artificial elements 31, Photoconductivity of semiconductors 31, Radiation detecting instruments 32, Protection against radiation 32, X-ray equipment 33, Atomic and molecular ions 35, Electron physics 35, Tables of nuclear data 35, Atomic energy levels 36. 6. Chemistry 36 Radioactive carbohydrates 36, Dextran as a substitute for blood plasma 37, Acidity and basicity in organic solvents 37, Interchangeability of fuel gases 38, Los Angeles "smog" 39, Infrared spectra of alcohols 39, Electrodeposi- tion 39, Development of analytical methods 40, Physical constants 42. 7. Mechanics 42 Turbulent flow 43, Turbulence at supersonic speeds 43, Dynamic properties of materials 43, High-frequency vibrations 44, Hearing loss 44, Physical properties by sonic methods 44, Water waves 46, Density currents 46, Precision weighing 46, Viscosity of gases 46, Evaporated thin films 47. -
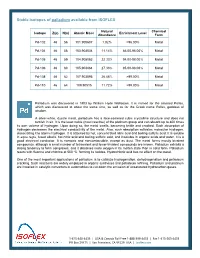
Stable Isotopes of Palladium Available from ISOFLEX
Stable isotopes of palladium available from ISOFLEX Natural Chemical Isotope Z(p) N(n) Atomic Mass Enrichment Level Abundance Form Pd-102 46 56 101.905607 1.02% >96.00% Metal Pd-104 46 58 103.904034 11.14% 86.00-98.00% Metal Pd-105 46 59 104.905083 22.33% 94.00-98.00% Metal Pd-106 46 60 105.903484 27.33% 95.00-98.00% Metal Pd-108 46 62 107.903895 26.46% >99.00% Metal Pd-110 46 64 109.90515 11.72% >99.00% Metal Palladium was discovered in 1803 by William Hyde Wollaston. It is named for the asteroid Pallas, which was discovered at about the same time, as well as for the Greek name Pallas, goddess of wisdom. A silver-white, ductile metal, palladium has a face-centered cubic crystalline structure and does not tarnish in air. It is the least noble (most reactive) of the platinum group and can absorb up to 800 times its own volume of hydrogen. Upon doing so, the metal swells, becoming brittle and cracked. Such absorption of hydrogen decreases the electrical conductivity of the metal. Also, such absorption activates molecular hydrogen, dissociating it to atomic hydrogen. It is attacked by hot, concentrated nitric acid and boiling sulfuric acid. It is soluble in aqua regia, fused alkalis, hot nitric acid and boiling sulfuric acid, and insoluble in organic acids and water. It is a good electrical conductor. It is nontoxic and noncombustible, except as dust. The metal forms mostly bivalent compounds, although a small number of tetravalent and fewer trivalent compounds are known. -

New Technologies for 211At Targeted Α-Therapy Research Using 211Rn and 209At
New technologies for 211At targeted α-therapy research using 211Rn and 209At Jason Raymond Crawford BSc, University of British Columbia, 2007 MSc, University of Victoria, 2010 A dissertation submitted in partial fulfilment of the requirements for the degree of Doctorate of Philosophy in the Department of Physics and Astronomy c Jason Raymond Crawford, 2016 University of Victoria All rights reserved. This dissertation may not be reproduced in whole or in part by photocopy or other means, without the permission of the author. ii New technologies for 211At targeted α-therapy research using 211Rn and 209At by Jason Raymond Crawford BSc, University of British Columbia, 2007 MSc, University of Victoria, 2010 Supervisory Committee Dr. Thomas J Ruth, Co-Supervisor Department of Physics and Astronomy Dr. Andrew Jirasek, Co-Supervisor Department of Physics and Astronomy Dr. Wayne Beckham, Committee Member Department of Physics and Astronomy Dr. Dean Karlen, Committee Member Department of Physics and Astronomy Dr. Julian Lum, Outside Member Department of Biochemistry & Microbiology iii Abstract The most promising applications for targeted α-therapy with astatine-211 (211At) in- clude treatments of disseminated microscopic disease, the major medical problem for cancer treatment. The primary advantages of targeted α-therapy with 211At are that the α-particle radiation is densely ionizing, translating to high relative biological effectiveness (RBE), and short-range, minimizing damage to surrounding healthy tissues. In addition, theranostic imaging with 123I surrogates has shown promise for developing new therapies with 211At and translating them to the clinic. Currently, Canada does not have a way of producing 211At by conventional methods because it lacks α-particle accelerators with necessary beam energy and intensity. -

Lawrence Berkeley National Laboratory Recent Work
Lawrence Berkeley National Laboratory Recent Work Title PART A. AN X-RAY SPECTROMETER FOR USE IN RADIOACTIVITY MEASUREMENTS, THE L X- RAYS OF NEPTUNIUM AND PLUTONIUM. PART B. SOME LIGHTER ISOTOPES OF ASTATINE Permalink https://escholarship.org/uc/item/9ss952mr Author Barton, G.W. Publication Date 1950-05-02 eScholarship.org Powered by the California Digital Library University of California ·. l '; UCRL~67o · cy 2 UNIVERSITY OF CALIFORNIA TWO-WEEK LOAN COPY This is a Library Circulating Copy which may be borrowed for two weeks. For a personal retention copy, call Tech. Info. Division, Ext. 5545 BERKELEY, CALIFORNIA DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. • .\ UCRL-670 No Distribution II]ECLASSIF~ED UNIVERSITY OF CALIFORNIA Radiation Laboratory Contract No. -

Cosmogenic Production As a Background in Searching For
Cosmogenic Production as a Background in Searching for Rare Physics Processes D.-M. Mei a , Z.-B. Yin a,b,c,1, S. R. Elliott d aDepartment of Physics, The University of South Dakota, Vermillion, South Dakota 57069 bInstitute of Particle Physics, Huazhong Normal University, Wuhan 430079, China cKey Laboratory of Quark & Lepton Physics (Huazhong Normal University), Ministry of Education, China dLos Alamos National Laboratory, Los Alamos, New Mexico 87545 Abstract We revisit calculations of the cosmogenic production rates for several long-lived iso- topes that are potential sources of background in searching for rare physics processes such as the detection of dark matter and neutrinoless double-beta decay. Using up- dated cosmic-ray neutron flux measurements, we use TALYS 1.0 to investigate the cosmogenic activation of stable isotopes of several detector targets and find that the cosmogenic isotopes produced inside the target materials and cryostat can result in large backgrounds for dark matter searches and neutrinoless double-beta decay. We use previously published low-background HPGe data to constrain the production of 3H on the surface and the upper limit is consistent with our calculation. We note that cosmogenic production of several isotopes in various targets can generate po- arXiv:0903.2273v1 [nucl-ex] 12 Mar 2009 tential backgrounds for dark matter detection and neutrinoless double-beta decay with a massive detector, thus great care should be taken to limit and/or deal with the cosmogenic activation of the targets. Key words: Cosmogenic activation, Dark matter detection, Double-beta decay PACS: 13.85.Tp, 23.40-s, 25.40.Sc, 28.41.Qb, 95.35.+d, 29.40.Wk Email address: [email protected] (D.-M. -

(12) United States Patent (10) Patent No.: US 8,625,731 B2 Holden Et Al
USOO8625731B2 (12) United States Patent (10) Patent No.: US 8,625,731 B2 Holden et al. (45) Date of Patent: Jan. 7, 2014 (54) COMPACT NEUTRONGENERATOR FOR (51) Int. Cl. MEDICAL AND COMMERCIAL SOTOPE G2G 4/02 (2006.01) PRODUCTION, FISSION PRODUCT G2G 4/00 (2006.01) PURIFICATION AND CONTROLLED (52) U.S. Cl. GAMMA REACTIONS FOR DIRECT USPC ............................ 376/108: 376/112:376/156 ELECTRIC POWER GENERATION (58) Field of Classification Search USPC .......................... 376/157, 108,156, 171, 172 (76) Inventors: Charles S. Holden, San Francisco, CA See application file for complete search history. (US); Robert E. Schenter, Portland, OR (US) (56) References Cited *) Notice: Subject to anyy disclaimer, the term of this U.S. PATENT DOCUMENTS patent is extended or adjusted under 35 3,748,226 A * 7/1973 Ribe et al. ..................... 376,124 U.S.C. 154(b) by 961 days. 3,778,627 A * 12/1973 Carpenter ...... ... 376, 192 4,749,540 A * 6/1988 Bogartet al. .. ... 376/133 (21) Appl. No.: 12/296,844 4,997,619 A * 3/1991 Pettus ............ ... 376,288 2004/02284.33 A1* 1 1/2004 Magill et al. ... 376/347 (22) PCT Filed: Apr. 13, 2007 2005/022O248 A1* 10, 2005 Ritter ...... ... 376/190 2008/O144762 A1* 6/2008 Holden et al. ..... ... 376/416 (86). PCT No.: PCT/US2OOTAO66668 * cited by examiner S371 (c)(1), (2), (4) Date: Oct. 10, 2008 Primary Examiner — Jack W Keith Assistant Examiner — Sean P Burke (87) PCT Pub. No.: WO2008/060663 (74) Attorney, Agent, or Firm — Craig M. Stainbrook; PCT Pub. Date: May 22, 2008 Stainbrook & Stainbrook, LLP (65) Prior Publication Data (57) ABSTRACT A neutron generator and isotope production apparatus and US 2011 FO268237 A1 Nov.