Roles of Panx1 Channels in Larval Zebrafish: from Genes to Visual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Browsing Genes and Genomes with Ensembl
The Bioinformatics Roadshow Tórshavn, The Faroe Islands 28-29 November 2012 BROWSING GENES AND GENOMES WITH ENSEMBL EXERCISES AND ANSWERS 1 BROWSER 3 BIOMART 8 VARIATION 13 COMPARATIVE GENOMICS 18 2 Note: These exercises are based on Ensembl version 69 (October 2012). After in future a new version has gone live, version 69 will still be available at http://e69.ensembl.org/. If your answer doesn’t correspond with the given answer, please consult the instructor. ______________________________________________________________ BROWSER ______________________________________________________________ Exercise 1 – Exploring a gene (a) Find the human F9 (coagulation factor IX) gene. On which chromosome and which strand of the genome is this gene located? How many transcripts (splice variants) have been annotated for it? (b) What is the longest transcript? How long is the protein it encodes? Has this transcript been annotated automatically (by Ensembl) or manually (by Havana)? How many exons does it have? Are any of the exons completely or partially untranslated? (c) Have a look at the external references for ENST00000218099. What is the function of F9? (d) Is it possible to monitor expression of ENST00000218099 with the ILLUMINA HumanWG_6_V2 microarray? If so, can it also be used to monitor expression of the other two transcripts? (e) In which part (i.e. the N-terminal or C-terminal half) of the protein encoded by ENST00000218099 does its peptidase activity reside? (f) Have any missense variants been discovered for the protein encoded by ENST00000218099? (g) Is there a mouse orthologue predicted for the human F9 gene? (h) If you have yourself a gene of interest, explore what information Ensembl displays about it! ______________________________________________________________ Answer (a) 8 Go to the Ensembl homepage (http://www.ensembl.org/). -

Polyhexamethylene Guanidine Phosphate Damages Tight Junctions and the F-Actin Architecture by Activating Calpain-1 Via the P2RX7/Ca2+ Signaling Pathway
cells Article Polyhexamethylene Guanidine Phosphate Damages Tight Junctions and the F-Actin Architecture by Activating Calpain-1 via the P2RX7/Ca2+ Signaling Pathway Sun Woo Jin y, Gi Ho Lee y, Hoa Thi Pham, Jae Ho Choi and Hye Gwang Jeong * College of Pharmacy, Chungnam National University, Daejeon 34134, Korea; [email protected] (S.W.J.); [email protected] (G.H.L.); [email protected] (H.T.P.); [email protected] (J.H.C.) * Correspondence: [email protected]; Tel.: +82-42-821-5936 These authors contributed equally to this work. y Received: 14 November 2019; Accepted: 22 December 2019; Published: 24 December 2019 Abstract: Polyhexamethylene guanidine phosphate (PHMG-p), a member of the polymeric guanidine family, has strong antimicrobial activity and may increase the risk of inflammation-associated pulmonary fibrosis. However, the effect of PHMG-p on the barrier function of the bronchial epithelium is unknown. Epithelial barrier functioning is maintained by tight junctions (TJs); damage to these TJs is the major cause of epithelial barrier breakdown during lung inflammation. The present study showed that, in BEAS-2B human bronchial epithelial cells, exposure to PHMG-p reduced the number of TJs and the E-cadherin level and impaired the integrity of the F-actin architecture. Furthermore, exposure to PHMG-p stimulated the calcium-dependent protease calpain-1, which breaks down TJs. However, treatment with the calpain-1 inhibitor, ALLN, reversed the PHMG-p-mediated impairment of TJs and the F-actin architecture. Furthermore, exposure to PHMG-p increased the intracellular Ca2+ level via P2X purinoreceptor 7 (P2RX7) and inhibition of P2RX7 abolished the PHMG-p-induced calpain-1 activity and protein degradation and increased the intracellular Ca2+ level. -

Novel Approach to Chronic Cough
Moksliniai darbai ir apžvalgos Novel approach to chronic cough NAUJAS POŽIŪRIS Į LĖTINĮ KOSULĮ LAIMA KONDRATAVIČIENĖ, KRISTINA BIEKŠIENĖ, SKAIDRIUS MILIAUSKAS Department of Pulmonology, Medical Academy, Lithuanian University of Health Sciences Summary. Cough is the most common symptom for which people seek medical advice. A multitude of reasons can cause it. In clinical practice, a new term “Cough hypersensitivity syndrome“ was proposed, which defines unaccountable reasons for cough and different groups of patients with chronic cough. Adenosine triphosphate (ATP) as a driver of chronic cough is the most important target in nowadays clinical trials. Extracellular ATP activates P2X purinoreceptor 3 (P2X3) receptor channels, which are expressed in sensory neurons. New treatment methods that block P2X3 receptors are being developed. Keywords: chronic cough, cough hypersensitivity syndrome, adenosine triphosphate, novel treatment options. Santrauka. Lėtinis kosulys yra dažniausias skundas, dėl kurio pacientai kreipiasi į gydytojus. Kosulį sukelia įvairios priežastys ir sutrikimai. Klinikinėje praktikoje vartojamas naujas terminas „Kosulio hiperjautrumo sindromas“, kuris apima neaiškos kilmės kosulio priežastis bei skirtingas pacientų, besiskundžiančių lėtiniu kosuliu, grupes. Adenozino trifosfatas (ATP), kaip vienas pagrindinių kosulį sukeliančių veiksnių, šiuo metu yra dažniausiai klinikiniuose tyrimuose tiriama cheminė medžiaga. ATP aktyvuoja P2X purino receptoriaus 3 (P2X3) jonų kanalus, kurie yra išreikšti jutiminiuose neuronuose. Nauji -

Sugar Causes Obesity and Metabolic Syndrome in Mice Independently of Sweet Taste
Am J Physiol Endocrinol Metab 319: E276–E290, 2020. First published June 23, 2020; doi:10.1152/ajpendo.00529.2019. RESEARCH ARTICLE Sugar causes obesity and metabolic syndrome in mice independently of sweet taste Ana Andres-Hernando,1 Masanari Kuwabara,1 X David J. Orlicky,2 Aurelie Vandenbeuch,3,4 Christina Cicerchi,1 Sue C. Kinnamon,3,4 Thomas E. Finger,4,5 X Richard J. Johnson,1 and X Miguel A. Lanaspa1 1Division of Renal Diseases and Hypertension, University of Colorado School of Medicine, University of Colorado, Aurora, Colorado; 2Department of Pathology, University of Colorado School of Medicine, University of Colorado, Aurora, Colorado; 3Department of Otolaryngology, University of Colorado School of Medicine, University of Colorado, Aurora, Colorado; 4Rocky Mountain Taste & Smell Center, University of Colorado School of Medicine, University of Colorado, Aurora, Colorado; and 5Department of Cell and Developmental Biology, University of Colorado School of Medicine, University of Colorado, Aurora, Colorado Submitted 5 December 2019; accepted in final form 16 June 2020 Andres-Hernando A, Kuwabara M, Orlicky DJ, Vandenbeuch caloric sweeteners has skyrocketed over the last several cen- A, Cicerchi C, Kinnamon SC, Finger TE, Johnson RJ, Lanaspa turies, from an intake (based on sales) of ~4 pounds per capita MA. Sugar causes obesity and metabolic syndrome in mice indepen- per year in 1700 to over 150 pounds per capita per year in 2000 dently of sweet taste. Am J Physiol Endocrinol Metab 319: E276– E290, 2020. First published June 23, 2020; doi:10.1152/ajpendo. (12). Today nearly 70% of processed foods and beverages in 00529.2019.—Intake of sugars, especially the fructose component, is US supermarkets contain these sweeteners, including many strongly associated with the development of obesity and metabolic foods that one might initially not consider to contain such syndrome, but the relative role of taste versus metabolism in driving additives (30). -
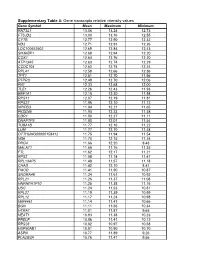
Gene Transcripts Relative Intensity Values
Supplementary Table 4: Gene transcripts relative intensity values Gene Symbol Mean Maximum Minimum RN7SL1 13.06 13.24 12.73 FTSJD2 13.00 13.16 12.55 CYTB 12.77 12.90 12.32 ND2 12.71 12.91 12.26 LOC100652902 12.69 12.84 12.43 SH3KBP1 12.68 12.94 12.20 COX1 12.64 12.76 12.20 ATP13A5 12.63 12.74 12.29 CCDC104 12.62 12.74 12.24 RPL41 12.58 12.66 12.36 TPT1 12.51 12.70 11.96 PTPRO 12.49 12.70 12.06 FN1 12.33 12.68 12.00 TLE1 12.26 12.43 11.93 EEF1A1 12.15 12.30 11.98 RPS11 12.07 12.19 11.81 RPS27 11.96 12.10 11.72 NPIPB3 11.94 12.21 11.65 FKSG49 11.94 12.23 11.58 CDR1 11.90 12.27 11.11 DNAPTP3 11.80 12.07 11.54 TUBA1B 11.77 12.16 11.22 LUM 11.77 12.10 11.48 OTTHUMG00000158412 11.75 11.94 11.54 ND6 11.70 12.15 11.24 PRG4 11.66 12.20 9.43 MALAT1 11.65 11.76 11.35 FTL 11.62 12.17 11.21 RPS2 11.58 11.74 11.47 RPL13AP5 11.48 11.57 11.18 CHAD 11.42 12.10 8.41 FMOD 11.41 11.90 10.87 SNORA48 11.34 11.61 10.92 RPL21 11.26 11.37 11.08 HNRNPA1P10 11.25 11.38 11.16 UBC 11.24 11.53 10.81 RPL27 11.19 11.39 10.99 RPL12 11.17 11.24 10.98 MIR4461 11.14 11.47 10.66 BGN 11.11 11.66 10.34 HTRA1 11.01 11.87 9.65 NEAT1 10.93 11.38 10.23 PRELP 10.86 11.41 10.12 RPS28 10.82 10.97 10.58 HSP90AB1 10.81 10.90 10.70 ASPN 10.77 11.89 9.26 PLA2G2A 10.76 11.47 9.56 H3F3A 10.69 10.86 10.41 EEF1G 10.69 10.94 10.11 C6orf48 10.64 11.02 10.35 UBA52 10.58 10.75 10.08 CTGF 10.57 11.33 9.66 MGP 10.57 11.22 10.09 YBX1 10.56 10.79 10.01 MT1X 10.56 11.89 9.88 NPC2 10.55 10.80 10.12 LAPTM4A 10.51 10.78 10.18 ITM2B 10.51 10.68 10.34 IBSP 10.50 11.50 7.17 NPIPB5 10.50 10.73 10.26 TUBA1A -

This Item Is the Archived Peer-Reviewed Author-Version Of
This item is the archived peer-reviewed author-version of: Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function Reference: Houbrechts Anne M., Vergauwen Lucia, Bagci Enise, Van houcke Jolien, Heijlen Marjolein, Kulemeka Bernard, Hyde David R., Knapen Dries, Darras Veerle M..- Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function Molecular and cellular endocrinology - ISSN 0303-7207 - 424(2016), p. 81-93 Full text (Publishers DOI): http://dx.doi.org/doi:10.1016/j.mce.2016.01.018 To cite this reference: http://hdl.handle.net/10067/1314550151162165141 Institutional repository IRUA 1 Deiodinase knockdown affects zebrafish eye development at the level 2 of gene expression, morphology and function. 3 Anne M. Houbrechts1*, Lucia Vergauwen2*, Enise Bagci2,3, Jolien Van houcke1, Marjolein 4 Heijlen1, Bernard Kulemeka4, David R. Hyde4, Dries Knapen2, Veerle M. Darras1 5 1 Laboratory of Comparative Endocrinology, Department of Biology, Division of 6 Animal Physiology and Neurobiology, KU Leuven, 3000 Leuven, Belgium 7 2 Zebrafishlab, Veterinary Physiology and Biochemistry, Department of Veterinary 8 Sciences, University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Belgium 9 3 Systemic Physiological & Ecotoxicological Research, Department of Biology, 10 University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Belgium 11 4 Department of Biological Sciences and Center for Zebrafish Research, University of 12 Notre Dame, Notre Dame, -

Ion Channels and Transporters in Lymphocyte Function and Immunity
REVIEWS Ion channels and transporters in lymphocyte function and immunity Stefan Feske1, Edward Y. Skolnik2 and Murali Prakriya3 Abstract | Lymphocyte function is regulated by a network of ion channels and transporters in the plasma membrane of B and T cells. These proteins modulate the cytoplasmic concentrations of diverse cations, such as calcium, magnesium and zinc ions, which function as second messengers to regulate crucial lymphocyte effector functions, including cytokine production, differentiation and cytotoxicity. The repertoire of ion-conducting proteins includes calcium release-activated calcium (CRAC) channels, P2X receptors, transient receptor potential (TRP) channels, potassium channels, chloride channels and magnesium and zinc transporters. This Review discusses the roles of ion conduction pathways in lymphocyte function and immunity. Ion channels and ion transporters function as gateways Store-operated calcium channels Ion channels 2+ Pore-forming transmembrane for charged ions that cannot freely diffuse across lipid Ca is a well-established second messenger in lympho proteins that enable the flow of membrane barriers. They regulate the intracellular cytes that regulates proliferation, gene expression, motil- ions down an electrochemical concentration of various ions, such as calcium (Ca2+), ity and other functions. Similarly to in other mammalian gradient. magnesium (Mg2+) and zinc (Zn2+). The movement of cell types, the intracellular Ca2+ concentration in unstim- Ion transporters these cations across the plasma membrane depends on ulated B and T cells is maintained at ~50–100 nM, which 4 2+ Pore-forming transmembrane electrical gradients that are maintained in turn by potas- is ~10 -fold lower than the Ca concentration in the proteins that carry ions sium (K+), sodium (Na+) and chloride (Cl−) channels. -

A Brain-Derived Neurotrophic Factor
www.nature.com/scientificreports OPEN A Brain-Derived Neurotrophic Factor Mimetic Is Sufcient to Restore Cone Photoreceptor Visual Received: 17 January 2017 Accepted: 25 August 2017 Function in an Inherited Blindness Published: xx xx xxxx Model Conor Daly1, Lisa Shine1, Theresa Hefernan1, Sudhakar Deeti1, Alison L. Reynolds 1, John J. O’Connor1, Eugène T. Dillon 1,2, David J. Dufy 3,5, Walter Kolch 1,3,4, Gerard Cagney1 & Breandán N. Kennedy 1 Controversially, histone deacetylase inhibitors (HDACi) are in clinical trial for the treatment of inherited retinal degeneration. Utilizing the zebrafsh dyeucd6 model, we determined if treatment with HDACi can rescue cone photoreceptor-mediated visual function. dye exhibit defective visual behaviour and retinal morphology including ciliary marginal zone (CMZ) cell death and decreased photoreceptor outer segment (OS) length, as well as gross morphological defects including hypopigmentation and pericardial oedema. HDACi treatment of dye results in signifcantly improved optokinetic (OKR) (~43 fold, p < 0.001) and visualmotor (VMR) (~3 fold, p < 0.05) responses. HDACi treatment rescued gross morphological defects and reduced CMZ cell death by 80%. Proteomic analysis of dye eye extracts suggested BDNF-TrkB and Akt signaling as mediators of HDACi rescue in our dataset. Co- treatment with the TrkB antagonist ANA-12 blocked HDACi rescue of visual function and associated Akt phosphorylation. Notably, sole treatment with a BDNF mimetic, 7,8-dihydroxyfavone hydrate, signifcantly rescued dye visual function (~58 fold increase in OKR, p < 0.001, ~3 fold increase in VMR, p < 0.05). In summary, HDACi and a BDNF mimetic are sufcient to rescue retinal cell death and visual function in a vertebrate model of inherited blindness. -
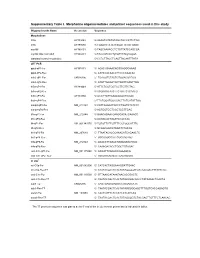
Supplementary Table I. Morpholino Oligonucleotides and Primer Sequences Used in This Study
Supplementary Table I. Morpholino oligonucleotides and primer sequences used in this study Oligonucleotide Name Accession Sequence Morpholinos tlr5a AY389449 5'-AAAGTGTATGTAGCTGCCATTCTGG tlr5b AY389450 5'-TGAATGTATATCCCATTCTGTGAGC myd88 AY388401 5'-TAGCAAAACCTCTGTTATCCAGCGA myd88 5bp mismatch AY388401 5'-TAcCAtAACCTgTGTTATCgAGgGA standard control morpholino 5'-CCTCTTACCTCAGTTACAATTTATA qRT-PCR ppial-qP1-Fw AY391451 5’- ACACTGAAACACGGAGGCAAAG ppial-qP2-Rev 5’- CATCCACAACCTTCCCGAACAC irak3-qP1-Fw CK026195 5’- TGAGGTCTACTGTGGACGATGG irak3-qP2-Rev 5’- ATGTTAGGATGCTGGTTGAGTTGG tlr5a-qP1-Fw AY389449 5’-ATTCTGGTGGTGCTTGTTGTAG tlr5a-qP2-Rev 5’-ACGAGGTAACTTCTGTTCTCAATG tlr5b-qP3-Fw AY389450 5’-GCGTTGTTGAAGAGGCTGGAC tlr5b-qP4-Rev 5’-TTCTGGATGGCCACTTCTCATATTGG mmp9-qP3-Fw NM_213123 5’-CATTAAAGATGCCCTGATGTATCCC mmp9-qP4-Rev 5’-AGTGGTGGTCCGTGGTTGAG il1b-qP1-Fw NM_212844 5’-GAACAGAATGAAGCACATCAAACC il1b-qP2-Rev 5’-ACGGCACTGAATCCACCAC il8-qP1-Fw XM_001342570 5’-TGTGTTATTGTTTTCCTGGCATTTC il8-qP2-Rev 5’-GCGACAGCGTGGATCTACAG ifn1-qP3-Fw NM_207640 5’- TTAATACACGCAAAGATGAGAACTC ifn1-qP4-Rev 5’- GCCAAGCCATTCGCAAGTAG tnfa-qP5-Fw NM_212829 5’- AGACCTTAGACTGGAGAGATGAC tnfa-qP6-Rev 5’- CAAAGACACCTGGCTGTAGAC cxcl-C1c-qP1-Fw NM_001115060 5’- GGCATTCACACCCAAAGCG cxcl-C1c-qP2_Rev 5’- GCGAGCACGATTCACGAGAG * In situ ccl-C5a-Fw NM_001082906 5’- CATCACTAGGAAAGGATTGAAC ccl-C5a-Rev-T7 5’- TAATACGACTCACTATAGGGGATGTCAAAGACTTTATTCAC cxcl-C1c-Fw NM_001115060 5’- GTTAAACATAAATAACACCGACTC cxcl-C1c-Rev-T7 5’- TAATACGACTCACTATAGGGACACCCTATAAAACTGAGTA irak3-Fw CK026195 5’- CAGTGAGAGAGGCATGAAACATC -

Inhibition of P2X4R Attenuates White Matter Injury in Mice After
Fu et al. Journal of Neuroinflammation (2021) 18:184 https://doi.org/10.1186/s12974-021-02239-3 RESEARCH Open Access Inhibition of P2X4R attenuates white matter injury in mice after intracerebral hemorrhage by regulating microglial phenotypes Xiongjie Fu†, Guoyang Zhou†, Xinyan Wu†, Chaoran Xu, Hang Zhou, Jianfeng Zhuang, Yucong Peng, Yang Cao, Hanhai Zeng, Yin Li, Jianru Li, Liansheng Gao, Gao Chen* , Lin Wang* and Feng Yan* Abstract Background: White matter injury (WMI) is a major neuropathological event associated with intracerebral hemorrhage (ICH). P2X purinoreceptor 4 (P2X4R) is a member of the P2X purine receptor family, which plays a crucial role in regulating WMI and neuroinflammation in central nervous system (CNS) diseases. Our study investigated the role of P2X4R in the WMI and the inflammatory response in mice, as well as the possible mechanism of action after ICH. Methods: ICH was induced in mice via collagenase injection. Mice were treated with 5-BDBD and ANA-12 to inhibit P2X4R and tropomyosin-related kinase receptor B (TrkB), respectively. Immunostaining and quantitative polymerase chain reaction (qPCR) were performed to detect microglial phenotypes after the inhibition of P2X4R. Western blots (WB) and immunostaining were used to examine WMI and the underlying molecular mechanisms. Cylinder, corner turn, wire hanging, and forelimb placement tests were conducted to evaluate neurobehavioral function. Results: After ICH, the protein levels of P2X4R were upregulated, especially on day 7 after ICH, and were mainly located in the microglia. The inhibition of P2X4R via 5-BDBD promoted neurofunctional recovery after ICH as well as the transformation of the pro-inflammatory microglia induced by ICH into an anti-inflammatory phenotype, and attenuated ICH-induced WMI. -

Supplementary Information
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is © The Royal Society of Chemistry 2014 Supplementary Information High-throughput synthesis of stable isotope-labeled transmembrane proteins for targeted transmembrane proteomics using a wheat germ cell-free protein synthesis system Nobuaki Takemori a*, Ayako Takemori a, Kazuhiro Matsuoka ab, Ryo Morishita c, Natsuki Matsushita d, Masato Aoshima e, Hiroyuki Takeda ab, Tatsuya Sawasaki ab, Yaeta Endo b, and Shigeki Higashiyama a a Proteo-Science Center, Ehime University, Ehime, Japan b Cell-Free Science and Technology Research Center, Ehime University, Ehime, Japan, c CellFree Sciences Co., Ltd., Ehime, Japan, d Translational Research Center, Ehime University Hospital, Ehime, Japan, e K.K. AB SCIEX, Tokyo, Japan * Corresponding author E-mail: [email protected] Contents • Experimental Procedure • Supplementary Results • Supplementary Figures (5 figures) Fig. S1: Highly effective incorporation of stable isotope (SI)-labeled amino acids in a bilayer cell-free system. Fig. S2: A workflow diagram for the bilayer cell-free synthesis of transmembrane proteins. Fig. S3: SDS-PAGE images of mouse transmembrane proteins. Fig. S4: Western blot analysis of mouse GRIA3 in six brain regions. Fig. S5: Expression profiles of endogenous neurotransmitter receptors in six brain regions. • Supplementary Tables (7 tables; see “Supplementary Table.xlsx”) 1. Experimental Procedure 1.1 Concurrent synthesis of SI-labeled TMPs using WG-CFS We selected 263 cDNA clones encoding TMPs of interest from the Functional Annotation of Mouse (FANTOM) full-length cDNA library (DNAFORM, Yokohama, Japan). Split-primer PCR was performed using specific primers to produce DNA templates for in vitro transcription, as described previously.24 A peptide tag sequence (MGPGGRAIIIRAAQAGTVR) was designed to measure the absolute amount of synthesized TMPs using the tryptic fragment AIIIR; the specific nucleotide sequence of the tag peptide was fused to each targeted TMP cDNA at the 5ʹ end. -

Supplementary Data
SUPPLEMENTARY METHODS 1) Characterisation of OCCC cell line gene expression profiles using Prediction Analysis for Microarrays (PAM) The ovarian cancer dataset from Hendrix et al (25) was used to predict the phenotypes of the cell lines used in this study. Hendrix et al (25) analysed a series of 103 ovarian samples using the Affymetrix U133A array platform (GEO: GSE6008). This dataset comprises clear cell (n=8), endometrioid (n=37), mucinous (n=13) and serous epithelial (n=41) primary ovarian carcinomas and samples from 4 normal ovaries. To build the predictor, the Prediction Analysis of Microarrays (PAM) package in R environment was employed (http://rss.acs.unt.edu/Rdoc/library/pamr/html/00Index.html). When more than one probe described the expression of a given gene, we used the probe with the highest median absolute deviation across the samples. The dataset from Hendrix et al. (25) and the dataset of OCCC cell lines described in this manuscript were then overlaid on the basis of 11536 common unique HGNC gene symbols. Only the 99 primary ovarian cancers samples and the four normal ovary samples were used to build the predictor. Following leave one out cross-validation, a predictor based upon 126 genes was able to identify correctly the four distinct phenotypes of primary ovarian tumour samples with a misclassification rate of 18.3%. This predictor was subsequently applied to the expression data from the 12 OCCC cell lines to determine the likeliest phenotype of the OCCC cell lines compared to primary ovarian cancers. Posterior probabilities were estimated for each cell line in comparison to the following phenotypes: clear cell, endometrioid, mucinous and serous epithelial.