2014 SNA Research Conference Proceedings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RECORDS of the HAWAII BIOLOGICAL SURVEY for 1995 Part 2: Notes1
RECORDS OF THE HAWAII BIOLOGICAL SURVEY FOR 1995 Part 2: Notes1 This is the second of two parts to the Records of the Hawaii Biological Survey for 1995 and contains the notes on Hawaiian species of plants and animals including new state and island records, range extensions, and other information. Larger, more compre- hensive treatments and papers describing new taxa are treated in the first part of this Records [Bishop Museum Occasional Papers 45]. New Hawaiian Pest Plant Records for 1995 PATRICK CONANT (Hawaii Dept. of Agriculture, Plant Pest Control Branch, 1428 S King St, Honolulu, HI 96814) Fabaceae Ulex europaeus L. New island record On 6 October 1995, Hawaii Department of Land and Natural Resources, Division of Forestry and Wildlife employee C. Joao submitted an unusual plant he found while work- ing in the Molokai Forest Reserve. The plant was identified as U. europaeus and con- firmed by a Hawaii Department of Agriculture (HDOA) nox-A survey of the site on 9 October revealed an infestation of ca. 19 m2 at about 457 m elevation in the Kamiloa Distr., ca. 6.2 km above Kamehameha Highway. Distribution in Wagner et al. (1990, Manual of the flowering plants of Hawai‘i, p. 716) listed as Maui and Hawaii. Material examined: MOLOKAI: Molokai Forest Reserve, 4 Dec 1995, Guy Nagai s.n. (BISH). Melastomataceae Miconia calvescens DC. New island record, range extensions On 11 October, a student submitted a leaf specimen from the Wailua Houselots area on Kauai to PPC technician A. Bell, who had the specimen confirmed by David Lorence of the National Tropical Botanical Garden as being M. -

SAPIA NEWS No. 47 Page 2
Plant Protection Research SAPIA NEWS January 2018 SOUTHERN AFRICAN PLANT INVADERS ATLAS No. 47 Newsletter of the Southern African Plant Invaders Atlas, an initiative of the Weeds Research Division of Plant Protection Research, an institute within the Agricultural Research Council (ARC) Weed alerts—the deceitful charmer and spiny breeches! 1 2 Inside this issue: Weed alerts 1 Red sage (Salvia coccinea) 2–3 a potential invader Bear’s breeches 4–5 (Acanthus polystachius) a potential invader Photo: Claude Moshobane Photo: Alan Urban The South African National Biodiversity Institute (SANBI), Directorate: Biological Invasions (DBI), has issued two new weed alerts—Red sage (Salvia coccinea) (photo 1) and Bear’s Invasive torch cactus and breeches (Acanthus polystachius) (photo 2). The public can assist SANBI-DBI by sending 6–8 look-alikes in South Africa locality information of these species which can help assess their invasion status. Another torch cactus starts invading! Editor and SAPIA co-ordinator: Argentine giant cactus (Trichocereus Lesley Henderson candicans or Echinopsis candicans) ARC-PPRI is starting to invade the karoo around Weeds Research Division the town of Prince Albert. stationed at SANBI Private Bag X101 Pretoria Richard Dean has observed plants 0001 along fence lines, under bushes and South Africa next to boulders, indicating bird- dispersal of seed. e-mail: [email protected] Tel: 012 843 5035 Fax: 012 804 3211 This species is one of a group of co- lumnar, ribbed cacti, commonly re- Articles and photos by Lesley Henderson ferred to as torch cacti. It is similar to unless otherwise acknowledged the very invasive Trichocereus spa- chianus, known simply as torch cac- SAPIA newsletters are posted at tus in South Africa but elsewhere ARC website: www.arc.agric.za and known as golden or white torch cac- Invasive Species Website: invasives.co.za tus. -

Oaks (Quercus Spp.): a Brief History
Publication WSFNR-20-25A April 2020 Oaks (Quercus spp.): A Brief History Dr. Kim D. Coder, Professor of Tree Biology & Health Care / University Hill Fellow University of Georgia Warnell School of Forestry & Natural Resources Quercus (oak) is the largest tree genus in temperate and sub-tropical areas of the Northern Hemisphere with an extensive distribution. (Denk et.al. 2010) Oaks are the most dominant trees of North America both in species number and biomass. (Hipp et.al. 2018) The three North America oak groups (white, red / black, and golden-cup) represent roughly 60% (~255) of the ~435 species within the Quercus genus worldwide. (Hipp et.al. 2018; McVay et.al. 2017a) Oak group development over time helped determine current species, and can suggest relationships which foster hybridization. The red / black and white oaks developed during a warm phase in global climate at high latitudes in what today is the boreal forest zone. From this northern location, both oak groups spread together southward across the continent splitting into a large eastern United States pathway, and much smaller western and far western paths. Both species groups spread into the eastern United States, then southward, and continued into Mexico and Central America as far as Columbia. (Hipp et.al. 2018) Today, Mexico is considered the world center of oak diversity. (Hipp et.al. 2018) Figure 1 shows genus, sub-genus and sections of Quercus (oak). History of Oak Species Groups Oaks developed under much different climates and environments than today. By examining how oaks developed and diversified into small, closely related groups, the native set of Georgia oak species can be better appreciated and understood in how they are related, share gene sets, or hybridize. -

Ethnobotany and Phytomedicine of the Upper Nyong Valley Forest in Cameroon
African Journal of Pharmacy and Pharmacology Vol. 3(4). pp. 144-150, April, 2009 Available online http://www.academicjournals.org/ajpp ISSN 1996-0816 © 2009 Academic Journals Full Length Research Paper Ethnobotany and phytomedicine of the upper Nyong valley forest in Cameroon T. Jiofack1*, l. Ayissi2, C. Fokunang3, N. Guedje4 and V. Kemeuze1 1Millennium Ecologic Museum, P. O Box 8038, Yaounde – Cameroon. 2Cameron Wildlife Consevation Society (CWCS – Cameroon), Cameroon. 3Faculty of Medicine and Biomedical Science, University of Yaounde I, Cameroon. 4Institute of Agronomic Research for Development, National Herbarium of Cameroon, Cameroon. Accepted 24 March, 2009 This paper presents the results of an assessment of the ethnobotanical uses of some plants recorded in upper Nyong valley forest implemented by the Cameroon wildlife conservation society project (CWCS). Forestry transects in 6 localities, followed by socio-economic study were conducted in 250 local inhabitants. As results, medicinal information on 140 plants species belonging to 60 families were recorded. Local people commonly use plant parts which included leaves, bark, seed, whole plant, stem and flower to cure many diseases. According to these plants, 8% are use to treat malaria while 68% intervenes to cure several others diseases as described on. There is very high demand for medicinal plants due to prevailing economic recession; however their prices are high as a result of prevailing genetic erosion. This report highlighted the need for the improvement of effective management strategies focusing on community forestry programmes and aims to encourage local people participation in the conservation of this forest heritage to achieve a sustainable plant biodiversity and conservation for future posterity. -

Reporton the Rare Plants of Puerto Rico
REPORTON THE RARE PLANTS OF PUERTO RICO tii:>. CENTER FOR PLANT CONSERVATION ~ Missouri Botanical Garden St. Louis, Missouri July 15, l' 992 ACKNOWLEDGMENTS The Center for Plant Conservation would like to acknowledge the John D. and Catherine T. MacArthur Foundation and the W. Alton Jones Foundation for their generous support of the Center's work in the priority region of Puerto Rico. We would also like to thank all the participants in the task force meetings, without whose information this report would not be possible. Cover: Zanthoxy7um thomasianum is known from several sites in Puerto Rico and the U.S . Virgin Islands. It is a small shrub (2-3 meters) that grows on the banks of cliffs. Threats to this taxon include development, seed consumption by insects, and road erosion. The seeds are difficult to germinate, but Fairchild Tropical Garden in Miami has plants growing as part of the Center for Plant Conservation's .National Collection of Endangered Plants. (Drawing taken from USFWS 1987 Draft Recovery Plan.) REPORT ON THE RARE PLANTS OF PUERTO RICO TABLE OF CONTENTS Acknowledgements A. Summary 8. All Puerto Rico\Virgin Islands Species of Conservation Concern Explanation of Attached Lists C. Puerto Rico\Virgin Islands [A] and [8] species D. Blank Taxon Questionnaire E. Data Sources for Puerto Rico\Virgin Islands [A] and [B] species F. Pue~to Rico\Virgin Islands Task Force Invitees G. Reviewers of Puerto Rico\Virgin Islands [A] and [8] Species REPORT ON THE RARE PLANTS OF PUERTO RICO SUMMARY The Center for Plant Conservation (Center) has held two meetings of the Puerto Rlco\Virgin Islands Task Force in Puerto Rico. -
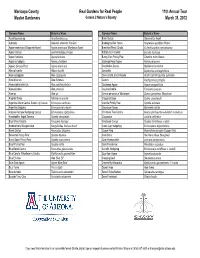
2012 Formatted Lists
Maricopa County Real Gardens for Real People 11th Annual Tour Master Gardeners Garden 2 Nature's Bounty March 31, 2012 Common Name Botanical Name Common Name Botanical Name Acanthocereus sp. Acanthocereus sp. Brain Cactus Stenocactus lloydii Adenium Adenium arabicum 'Fat Gun' Brakelights Red Yucca Hesperaloe parviflora 'Perpa' Agave americana 'Marginata Aurea' Agave americana 'Marginata Aurea' Branched Pencil Cholla Cylindropuntia ramosissima Agave Cactus Leuchtenbergia principis Brittlebush, Incienso Encelia farinosa Agave funkiana Agave funkiana Bunny Ears Prickly Pear Opuntia microdasys Agave schidigera Agave schidigera Cabbage Head Agave Agave parrasana Agave, Century Plant Agave americana Candelabra Cactus Myrtillocactus chohal Albuca humilis Albuca humilis Candelilla Euphorbia antisyphilitica Aloe cryptopoda Aloe cryptopoda Cane Cholla, Eve's Needle Austrocylindropuntia subulata Aloe ibitiensis Aloe ibitiensis Cardon Pachycereus pringlei Aloe porphyrostachys Aloe porphyrostachys Caribbean Agave Agave angustifolia Aloe prinslooii Aloe prinslooii Caudex Ocotillo Fouquieria purpusii Aloe sp. Aloe sp. Cereus peruvianus 'Monstrose' Cereus peruvianus 'Monstrose' Angelita Daisy Tetraneuris acaulis Chaparral Sage Salvia clevelandii Argentine Giant Cactus, Easter Lily Cactus Echinopsis candicans Chenille Prickly Pear Opuntia aciculata Argentine Saguaro Echinopsis terscheckii Chocolate Flower Berlandiera lyrata Arizona Rainbow Hedgehog Cactus Echinocereus rigidissimus Christmas Tree Cactus Austrocylindropuntia subulata f. monstrosa Arrastradillo, -

Foxato's Forum Magazine 13 °Volume Ufficiale
FOXATO'S FORUM MAGAZINE 13 °VOLUME UFFICIALE >> Premessa << Ciao amici miei. Torna il nostro caro magazine che rappresenta ancora una volta un riassunto della voce della famiglia che vuole ancora farsi sentire. Anche durante questa calda estate 2010 la musica non è mancata. Essa ci ha fatto compagnia in modo immenso su Foxato's Radio per tutti questi mesi. Abbiam assistito alla chiusura della vecchia e al ritorno della nuova stagione. Eventi per una maniera o per un'altra indimenticabili a dir poco . Quest'oggi il numero sarà incentrato principalmente su: -News musicali. -Scoperta di nuovi generi e alternative. -Album imperdibili. -Anticipazioni eventi nuova stagione. -Aggiornamento sezioni. Se siete pronti direi di partire subito con le news musicali. Ne sono molte da parte del sottoscritto e anche da parte del nostro amico aguzza_l'ingegno :-) :: Ringraziamenti iniziali a coloro che fan sì che la famiglia prosegua ancora al meglio :: :: NEWS MUSICALI ATTUALMENTE IN PROGRAMMAZIONE SU FOXATO’S RADIO :: Rileggendo lo scorso numero mi è venuta spontanea una riflessione su come siamo capaci (nel nostro piccolo) di rendere una marea di pezzi come delle vere e proprie colonne sonore di vita, in un'era dove si tende solamente a sputtanare la musica in tutti i sensi. E' un mondo che prosegue sempre di più verso una direzione disastrosa, eppure riesco ancora a considerarmi soddisfatto e fiero di ciò che con amore e passione conduco giornalmente su Foxato's Radio. E anche questo mese citiamo proprio la nostra oasi musicale come punto di partenza delle news musicali dato che non c'è miglior cosa che fare il punto della situazione su molteplici canzoni in programmazione. -

Humacao (787)850-3000
LIVEAPRIL/MAY 2009 VOL. 2 NUM. 2 LIFE IN PALMAS PHA & PALMAS DEL MAMothersR in Paradise HOMEOWNERS ASSOCIATION MAGAZINE PALMAS EXTREME WEEKENDERS [email protected] 1 Creamos los espacios de tus sueños • Material para pisos y paredes • Revestimiento de interiores y exteriores • Topes de cocina, baño, barra y más • Todo lo necesario para baños y cocinas CONTAMOS CON DECORADORAS Y ARQUITECTOS QUE CON MUCHO GUSTO LE AYUDARAN A DISENAR SU BANO O COCINA SIN COSTO ADICIONAL. PASE POR NUESTRO SHOWROOM... LE ESPERAMOS. 279 Ave. De Diego Puerto Nuevo www.marble-stone.com 2 [email protected] 2 [email protected] [email protected] 3 editor’s corner April & May are two of my favorite months. I guess this is so because it is a prelude to summer, my favorite time of the year. I have never abandoned the idea that summer is synonymous of beach time and vacation. Even if I don’t really go on vacations and I am working hard, during summer I feel like I am on vacation. This is especially true living in Palmas. During summer time Palmas become more vibrant and effervescent than ever, the water gets warmer and the beach is full with families and friends. With April come the Easter activities, PHA week, then mother’s day and...my birthday (no need for me to go into details). This year we will also have, very close to us, The Thunderbird’s air show; an exquisite concert called Duos de Amor with three important sopranos and tenors; and many other activities, please check the “Don’t Miss” it page. -

BYGL Newsletter | Buckeye Yard & Garden Online
BYGL Newsletter May 17, 2012 This is the 7th 2012 edition of the Buckeye Yard and Garden Line (BYGL). BYGL is developed from a Tuesday morning conference call of Extension Educators, Specialists, and other contributors in Ohio. Authors for 2015: Amanda Bennett, Pam Bennett, Joe Boggs, Jim Chatfield, Julie Crook, Erik Draper, Gary Gao, Denise Johnson, Jacqueline Kowalski, Ashley Kulhanek, Cindy Meyer, Amy Stone, Nancy Taylor, Marne Titchenell, Danae Wolfe, and Curtis Young. Plants of The Week » * Annual - Plectranthus 'Mona Lavendar' (Plectranthus spp.) Belonging to the mint family and having the common name SWEDISH IVY, this annual has incredible lavender blooms that show off above the plant. In Ohio gardens, it is an annual that grows with a rounded mound shape and gets around 18" tall and as wide. 'Mona Lavender' can be used in containers, bedding areas, and mixed in the perennial garden. In large containers, it makes an excellent filler plant. The rich purplish-green foliage adds color to containers; it is shiny and deep-green on top with a rich purple on the underside. The plant takes full sun or light shade and is considered deer resistant. The flower spikes have tubular lavender flowers that last for a long period of time, making deadheading almost unnecessary. For More Information: Missouri Botanical Garden Kemper Center for Home Gardening information on growing Plectranthus 'Mona Lavender' http://www.mobot.org/gardeninghelp/plantfinder/plant.asp *Perennial - Allium or Ornamental Onion (Allium spp.). In fact, don't deadhead the flowers until they completely lose their ornamental value. Many look great even dried in the garden. -

Illustrated Flora of East Texas Illustrated Flora of East Texas
ILLUSTRATED FLORA OF EAST TEXAS ILLUSTRATED FLORA OF EAST TEXAS IS PUBLISHED WITH THE SUPPORT OF: MAJOR BENEFACTORS: DAVID GIBSON AND WILL CRENSHAW DISCOVERY FUND U.S. FISH AND WILDLIFE FOUNDATION (NATIONAL PARK SERVICE, USDA FOREST SERVICE) TEXAS PARKS AND WILDLIFE DEPARTMENT SCOTT AND STUART GENTLING BENEFACTORS: NEW DOROTHEA L. LEONHARDT FOUNDATION (ANDREA C. HARKINS) TEMPLE-INLAND FOUNDATION SUMMERLEE FOUNDATION AMON G. CARTER FOUNDATION ROBERT J. O’KENNON PEG & BEN KEITH DORA & GORDON SYLVESTER DAVID & SUE NIVENS NATIVE PLANT SOCIETY OF TEXAS DAVID & MARGARET BAMBERGER GORDON MAY & KAREN WILLIAMSON JACOB & TERESE HERSHEY FOUNDATION INSTITUTIONAL SUPPORT: AUSTIN COLLEGE BOTANICAL RESEARCH INSTITUTE OF TEXAS SID RICHARDSON CAREER DEVELOPMENT FUND OF AUSTIN COLLEGE II OTHER CONTRIBUTORS: ALLDREDGE, LINDA & JACK HOLLEMAN, W.B. PETRUS, ELAINE J. BATTERBAE, SUSAN ROBERTS HOLT, JEAN & DUNCAN PRITCHETT, MARY H. BECK, NELL HUBER, MARY MAUD PRICE, DIANE BECKELMAN, SARA HUDSON, JIM & YONIE PRUESS, WARREN W. BENDER, LYNNE HULTMARK, GORDON & SARAH ROACH, ELIZABETH M. & ALLEN BIBB, NATHAN & BETTIE HUSTON, MELIA ROEBUCK, RICK & VICKI BOSWORTH, TONY JACOBS, BONNIE & LOUIS ROGNLIE, GLORIA & ERIC BOTTONE, LAURA BURKS JAMES, ROI & DEANNA ROUSH, LUCY BROWN, LARRY E. JEFFORDS, RUSSELL M. ROWE, BRIAN BRUSER, III, MR. & MRS. HENRY JOHN, SUE & PHIL ROZELL, JIMMY BURT, HELEN W. JONES, MARY LOU SANDLIN, MIKE CAMPBELL, KATHERINE & CHARLES KAHLE, GAIL SANDLIN, MR. & MRS. WILLIAM CARR, WILLIAM R. KARGES, JOANN SATTERWHITE, BEN CLARY, KAREN KEITH, ELIZABETH & ERIC SCHOENFELD, CARL COCHRAN, JOYCE LANEY, ELEANOR W. SCHULTZE, BETTY DAHLBERG, WALTER G. LAUGHLIN, DR. JAMES E. SCHULZE, PETER & HELEN DALLAS CHAPTER-NPSOT LECHE, BEVERLY SENNHAUSER, KELLY S. DAMEWOOD, LOGAN & ELEANOR LEWIS, PATRICIA SERLING, STEVEN DAMUTH, STEVEN LIGGIO, JOE SHANNON, LEILA HOUSEMAN DAVIS, ELLEN D. -

Effect of Heterotis Rotundifolia Crude Leaf Extract on Hb-S Erythrocyte Polymerization, Osmotic Fragility and Fe2+/Fe3+ Ratio
International Journal of Scientific & Engineering Research Volume 9, Issue 5, May-2018 1872 ISSN 2229-5518 Effect of Heterotis rotundifolia leaf extract on Hemoglobin-S (HbS) Polymerization, Osmotic fragility and Fe2+/Fe3+ ratio. Jane Adaeze Agwu, Augustine Amadikwa Uwakwe, Joyce Nornubari Nzor, Mfon Promise-Godsfavour Bobson AUTHORS Jane Adaeze Agwu BSc, Department of Biochemistry, University of Port Harcourt [email protected] 08162568028 *corresponding author Augustine Amadikwa Uwakwe PhD, Department of Biochemistry, University of Port Harcourt [email protected] Joyce Nornubari Nzor MSc, [email protected] 08025635584 *Mfon Promise-Godsfavour *Bobson (please correct) PhD , Department of Science Technology, School of Applied Sciences Akwa Ibom State Polytechnic [email protected] IJSER IJSER © 2018 http://www.ijser.org International Journal of Scientific & Engineering Research Volume 9, Issue 5, May-2018 1873 ISSN 2229-5518 ABSTRACT This study investigated the potential effect of Heterotis rotundifolia leaf extract on Human haemoglobin-S (HbS) erythrocyte on three criteria: gelation/polymerization rate, osmotic fragility and Fe2+/Fe3+ ratio. Stock solution concentrations of 10.0, 5.0, 3.3, 2.5 and 2.0 percent of the crude leaf extract of Heterotis rotundifolia were utilized for polymerization, osmotic fragility and Fe2+/Fe3+ ratio experiment. Spectrophotometric technique was used to measure the rate of gelation, level of osmotic fragility and Fe2+/Fe3+ ratio. Results show that the presence of increasing concentrations of Heterotis rotundifolia extract recorded a progressive decrease in HbS polymerization when compared to the control. Similarly, from the results of the osmotic fragility test, a reduction in HbS erythrocyte fragility was observed in the presence of Heterotis rotundifolia leaf extract. -

ASPECTOS BIOLÓGICOS DE Corytucha Gossypii (HEMIPTERA: TINGIDAE) COM FOLHAS DE MAMONA
UNIVERSIDADE ESTADUAL DA PARAÍBA CENTRO DE CIÊNCIAS BIOLÓGICAS E DA SAÚDE CURSO DE LICENCIATURA EM CIÊNCIAS BIOLÓGICAS Ana Lígia Aureliano de Lima e Silva ASPECTOS BIOLÓGICOS DE Corytucha gossypii (HEMIPTERA: TINGIDAE) COM FOLHAS DE MAMONA Campina Grande Dezembro 2012 Ana Lígia Aureliano de Lima e Silva ASPECTOS BIOLÓGICOS DE Corytucha gossypii (HEMIPTERA: TINGIDAE) COM FOLHAS DE MAMONA Trabalho de Conclusão de Curso (TCC) apresentado ao Curso de Graduação em Licenciatura em Ciências Biológicas da Universidade Estadual da Paraíba, em cumprimento à exigência para obtenção do grau de licenciado em Ciências Biológicas. Orientador(a): Carlos Alberto Domingues da Silva; Co orientador (a): Maria Avany Bezerra Gusmão F ICHA CATALOGRÁFICA ELABORADA PELA BIBLIOTECA CENTRAL – UEPB S586a Silva, Ana Lígia Aureliano de Lima e. Aspectos biológicos de Corytucha Gossypii (HEMIPTERA:TINGIDAE) com folhas de mamona. [manuscrito] / Ana Lígia Aureliano de Lima e Silva. – 2012. 15 f. : il. color. Digitado. Trabalho de Conclusão de Curso (Graduação em Ciências Biológicas) – Universidade Estadual da Paraíba, Centro de Ciências Biológicas e da Saúde, 2012. “Orientação: Prof. Dr. Carlos Alberto Domingues da Silva, EMBRAPA.” “Co- Orientação: Prof. Dra. Maria Anavy Beserra Gusmão, Departamento de Biologia.” 1. Mamona. 2. Corytucha gossypii. 3. Percevejo C. I. Título. CDD 21. ed. 570 ASPECTOS BIOLÓGICOS DE Corytucha gossypii (HEMIPTERA: TINGIDAE) COM FOLHAS DE MAMONA Trabalho de Conclusão de Curso (TCC) apresentado ao Curso de Graduação em Licenciatura em Ciências Biológicas da Universidade Estadual da Paraíba, em cumprimento à exigência para obtenção do grau de licenciado em Ciências Biológicas. APROVADA EM 14/12/2012 Aspectos biológicos de Corythucha gossypii (Hemiptera: Tingidae) em folhas de mamona RESUMO Corytucha gossypii (Fabricius) (Hemiptera: Tingidae) é responsável por ocasionar danos severos a diversas espécies vegetais.