Hopp04 Dinobrooding
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Chinese Archaeopterygian, Protarchaeopteryx Gen. Nov
A Chinese archaeopterygian, Protarchaeopteryx gen. nov. by Qiang Ji and Shu’an Ji Geological Science and Technology (Di Zhi Ke Ji) Volume 238 1997 pp. 38-41 Translated By Will Downs Bilby Research Center Northern Arizona University January, 2001 Introduction* The discoveries of Confuciusornis (Hou and Zhou, 1995; Hou et al, 1995) and Sinornis (Ji and Ji, 1996) have profoundly stimulated ornithologists’ interest globally in the Beipiao region of western Liaoning Province. They have also regenerated optimism toward solving questions of avian origins. In December 1996, the Chinese Geological Museum collected a primitive bird specimen at Beipiao that is comparable to Archaeopteryx (Wellnhofer, 1992). The specimen was excavated from a marl 5.5 m above the sediments that produce Sinornithosaurus and 8-9 m below the sediments that produce Confuciusornis. This is the first documentation of an archaeopterygian outside Germany. As a result, this discovery not only establishes western Liaoning Province as a center of avian origins and evolution, it provides conclusive evidence for the theory that avian evolution occurred in four phases. Specimen description Class Aves Linnaeus, 1758 Subclass Sauriurae Haeckel, 1866 Order Archaeopterygiformes Furbringer, 1888 Family Archaeopterygidae Huxley, 1872 Genus Protarchaeopteryx gen. nov. Genus etymology: Acknowledges that the specimen possesses characters more primitive than those of Archaeopteryx. Diagnosis: A primitive archaeopterygian with claviform and unserrated dentition. Sternum is thin and flat, tail is long, and forelimb resembles Archaeopteryx in morphology with three talons, the second of which is enlarged. Ilium is large and elongated, pubes are robust and distally fused, hind limb is long and robust with digit I reduced and dorsally migrated to lie in opposition to digit III and forming a grasping apparatus. -

LETTER Doi:10.1038/Nature14423
LETTER doi:10.1038/nature14423 A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings Xing Xu1,2*, Xiaoting Zheng1,3*, Corwin Sullivan2, Xiaoli Wang1, Lida Xing4, Yan Wang1, Xiaomei Zhang3, Jingmai K. O’Connor2, Fucheng Zhang2 & Yanhong Pan5 The wings of birds and their closest theropod relatives share a ratios are 1.16 and 1.08, respectively, compared to 0.96 and 0.78 in uniform fundamental architecture, with pinnate flight feathers Epidendrosaurus and 0.79 and 0.66 in Epidexipteryx), an extremely as the key component1–3. Here we report a new scansoriopterygid short humeral deltopectoral crest, and a long rod-like bone articu- theropod, Yi qi gen. et sp. nov., based on a new specimen from the lating with the wrist. Middle–Upper Jurassic period Tiaojishan Formation of Hebei Key osteological features are as follows. STM 31-2 (Fig. 1) is inferred Province, China4. Yi is nested phylogenetically among winged ther- to be an adult on the basis of the closed neurocentral sutures of the opods but has large stiff filamentous feathers of an unusual type on visible vertebrae, although this is not a universal criterion for maturity both the forelimb and hindlimb. However, the filamentous feath- across archosaurian taxa12. Its body mass is estimated to be approxi- ers of Yi resemble pinnate feathers in bearing morphologically mately 380 g, using an empirical equation13. diverse melanosomes5. Most surprisingly, Yi has a long rod-like The skull and mandible are similar to those of other scansoriopter- bone extending from each wrist, and patches of membranous tissue ygids, and to a lesser degree to those of oviraptorosaurs and some basal preserved between the rod-like bones and the manual digits. -

A New Raptorial Dinosaur with Exceptionally Long Feathering Provides Insights Into Dromaeosaurid flight Performance
ARTICLE Received 11 Apr 2014 | Accepted 11 Jun 2014 | Published 15 Jul 2014 DOI: 10.1038/ncomms5382 A new raptorial dinosaur with exceptionally long feathering provides insights into dromaeosaurid flight performance Gang Han1, Luis M. Chiappe2, Shu-An Ji1,3, Michael Habib4, Alan H. Turner5, Anusuya Chinsamy6, Xueling Liu1 & Lizhuo Han1 Microraptorines are a group of predatory dromaeosaurid theropod dinosaurs with aero- dynamic capacity. These close relatives of birds are essential for testing hypotheses explaining the origin and early evolution of avian flight. Here we describe a new ‘four-winged’ microraptorine, Changyuraptor yangi, from the Early Cretaceous Jehol Biota of China. With tail feathers that are nearly 30 cm long, roughly 30% the length of the skeleton, the new fossil possesses the longest known feathers for any non-avian dinosaur. Furthermore, it is the largest theropod with long, pennaceous feathers attached to the lower hind limbs (that is, ‘hindwings’). The lengthy feathered tail of the new fossil provides insight into the flight performance of microraptorines and how they may have maintained aerial competency at larger body sizes. We demonstrate how the low-aspect-ratio tail of the new fossil would have acted as a pitch control structure reducing descent speed and thus playing a key role in landing. 1 Paleontological Center, Bohai University, 19 Keji Road, New Shongshan District, Jinzhou, Liaoning Province 121013, China. 2 Dinosaur Institute, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California 90007, USA. 3 Institute of Geology, Chinese Academy of Geological Sciences, 26 Baiwanzhuang Road, Beijing 100037, China. 4 University of Southern California, Health Sciences Campus, BMT 403, Mail Code 9112, Los Angeles, California 90089, USA. -

Theory of the Growth and Evolution of Feather Shape
30JOURNAL R.O. PRUMOF EXPERIMENTAL AND S. WILLIAMSON ZOOLOGY (MOL DEV EVOL) 291:30–57 (2001) Theory of the Growth and Evolution of Feather Shape RICHARD O. PRUM* AND SCOTT WILLIAMSON Department of Ecology and Evolutionary Biology, and Natural History Museum, University of Kansas, Lawrence, Kansas 66045 ABSTRACT We present the first explicit theory of the growth of feather shape, defined as the outline of a pennaceous feather vane. Based on a reanalysis of data from the literature, we pro- pose that the absolute growth rate of the barbs and rachis ridges, not the vertical growth rate, is uniform throughout the follicle. The growth of feathers is simulated with a mathematical model based on six growth parameters: (1) absolute barb and rachis ridge growth rate, (2) angle of heli- cal growth of barb ridges, (3) initial barb ridge number, (4) new barb ridge addition rate, (5) barb ridge diameter, and (6) the angle of barb ramus expansion following emergence from the sheath. The model simulates growth by cell division in the follicle collar and, except for the sixth param- eter, does not account for growth by differentiation in cell size and shape during later keratiniza- tion. The model can simulate a diversity of feather shapes that correspond closely in shape to real feathers, including various contour feathers, asymmetrical feathers, and even emarginate prima- ries. Simulations of feather growth under different parameter values demonstrate that each pa- rameter can have substantial, independent effects on feather shape. Many parameters also have complex and redundant effects on feather shape through their influence on the diameter of the follicle, the barb ridge fusion rate, and the internodal distance. -

Implications for Predatory Dinosaur Macroecology and Ontogeny in Later Late Cretaceous Asiamerica
Canadian Journal of Earth Sciences Theropod Guild Structure and the Tyrannosaurid Niche Assimilation Hypothesis: Implications for Predatory Dinosaur Macroecology and Ontogeny in later Late Cretaceous Asiamerica Journal: Canadian Journal of Earth Sciences Manuscript ID cjes-2020-0174.R1 Manuscript Type: Article Date Submitted by the 04-Jan-2021 Author: Complete List of Authors: Holtz, Thomas; University of Maryland at College Park, Department of Geology; NationalDraft Museum of Natural History, Department of Geology Keyword: Dinosaur, Ontogeny, Theropod, Paleocology, Mesozoic, Tyrannosauridae Is the invited manuscript for consideration in a Special Tribute to Dale Russell Issue? : © The Author(s) or their Institution(s) Page 1 of 91 Canadian Journal of Earth Sciences 1 Theropod Guild Structure and the Tyrannosaurid Niche Assimilation Hypothesis: 2 Implications for Predatory Dinosaur Macroecology and Ontogeny in later Late Cretaceous 3 Asiamerica 4 5 6 Thomas R. Holtz, Jr. 7 8 Department of Geology, University of Maryland, College Park, MD 20742 USA 9 Department of Paleobiology, National Museum of Natural History, Washington, DC 20013 USA 10 Email address: [email protected] 11 ORCID: 0000-0002-2906-4900 Draft 12 13 Thomas R. Holtz, Jr. 14 Department of Geology 15 8000 Regents Drive 16 University of Maryland 17 College Park, MD 20742 18 USA 19 Phone: 1-301-405-4084 20 Fax: 1-301-314-9661 21 Email address: [email protected] 22 23 1 © The Author(s) or their Institution(s) Canadian Journal of Earth Sciences Page 2 of 91 24 ABSTRACT 25 Well-sampled dinosaur communities from the Jurassic through the early Late Cretaceous show 26 greater taxonomic diversity among larger (>50kg) theropod taxa than communities of the 27 Campano-Maastrichtian, particularly to those of eastern/central Asia and Laramidia. -

Magnificent Magpie Colours by Feathers with Layers of Hollow Melanosomes Doekele G
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb174656. doi:10.1242/jeb.174656 RESEARCH ARTICLE Magnificent magpie colours by feathers with layers of hollow melanosomes Doekele G. Stavenga1,*, Hein L. Leertouwer1 and Bodo D. Wilts2 ABSTRACT absorption coefficient throughout the visible wavelength range, The blue secondary and purple-to-green tail feathers of magpies are resulting in a higher refractive index (RI) than that of the structurally coloured owing to stacks of hollow, air-containing surrounding keratin. By arranging melanosomes in the feather melanosomes embedded in the keratin matrix of the barbules. barbules in more or less regular patterns with nanosized dimensions, We investigated the spectral and spatial reflection characteristics of vivid iridescent colours are created due to constructive interference the feathers by applying (micro)spectrophotometry and imaging in a restricted wavelength range (Durrer, 1977; Prum, 2006). scatterometry. To interpret the spectral data, we performed optical The melanosomes come in many different shapes and forms, and modelling, applying the finite-difference time domain (FDTD) method their spatial arrangement is similarly diverse (Prum, 2006). This has as well as an effective media approach, treating the melanosome been shown in impressive detail by Durrer (1977), who performed stacks as multi-layers with effective refractive indices dependent on extensive transmission electron microscopy of the feather barbules the component media. The differently coloured magpie feathers are of numerous bird species. He interpreted the observed structural realised by adjusting the melanosome size, with the diameter of the colours to be created by regularly ordered melanosome stacks acting melanosomes as well as their hollowness being the most sensitive as optical multi-layers. -

The Molecular Evolution of Feathers with Direct Evidence from Fossils
The molecular evolution of feathers with direct evidence from fossils Yanhong Pana,1, Wenxia Zhengb, Roger H. Sawyerc, Michael W. Penningtond, Xiaoting Zhenge,f, Xiaoli Wange,f, Min Wangg,h, Liang Hua,i, Jingmai O’Connorg,h, Tao Zhaoa, Zhiheng Lig,h, Elena R. Schroeterb, Feixiang Wug,h, Xing Xug,h, Zhonghe Zhoug,h,i,1, and Mary H. Schweitzerb,j,1 aChinese Academy of Sciences Key Laboratory of Economic Stratigraphy and Palaeogeography, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China; bDepartment of Biological Sciences, North Carolina State University, Raleigh, NC 27695; cDepartment of Biological Sciences, University of South Carolina, Columbia, SC 29205; dAmbioPharm Incorporated, North Augusta, SC 29842; eInstitute of Geology and Paleontology, Lingyi University, Lingyi City, 27605 Shandong, China; fShandong Tianyu Museum of Nature, Pingyi, 273300 Shandong, China; gCAS Key Laboratory of Vertebrate Evolution and Human Origins of the Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, 100044 Beijing, China; hCenter for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, 100044 Beijing, China; iCollege of Earth and Planetary Sciences, University of Chinese Academy of Sciences, 100049 Beijing, China; and jNorth Carolina Museum of Natural Sciences, Raleigh, NC 27601 Contributed by Zhonghe Zhou, December 15, 2018 (sent for review September 12, 2018; reviewed by Dominique G. Homberger and Chenxi Jia) Dinosaur fossils possessing integumentary appendages of various feathers in Anchiornis, barbules that interlock to form feather morphologies, interpreted as feathers, have greatly enhanced our vanes critical for flight have not been identified yet (12). -
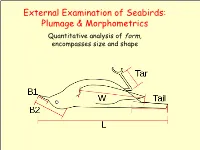
External Examination of Seabirds: Plumage & Morphometrics
External Examination of Seabirds: Plumage & Morphometrics Quantitative analysis of form, encompasses size and shape Seabird Topography ➢ Naming Conventions: • Parts of the body • Types of feathers (Harrison 1983) Types of Feathers Coverts: Rows bordering and overlaying the edges of the tail and wings on both the lower and upper sides of the body. Help streamline shape of the wings and tail and provide the bird with insulation. Feather Tracks ➢ Feathers are not attached randomly. • They occur in linear tracts called pterylae. • Spaces on bird's body without feather tracts are called apteria. • Densest area for feather tracks is head and neck. • Feathers arranged in distinct layers: contour feathers overlay down. Generic Pterylae Types of Feathers Contour feathers: outermost feathers. Define the color and shape of the bird. Contour feathers lie on top of each other, like shingles on a roof. Shed water, keeping body dry and insulated. Each contour feather controlled by specialized muscles which control their position, allowing the bird to keep the feathers in clean and neat condition. Specialized contour feathers used for flight: delineate outline of wings and tail. Types of Feathers Flight feathers – special contour feathers Define outline of wings and tail Long and stiff Asymmetrical those on wings are called remiges (singular remex) those on tail are called retrices (singular retrix) Types of Flight Feathers Remiges: Largest contour feathers (primaries / secondaries) Responsible for supporting bird during flight. Attached by ligaments or directly to the wing bone. Types of Flight Feathers Flight feathers – special contour feathers Rectrices: tail feathers provide flight stability and control. Connected to each other by ligaments, with only the inner- most feathers attached to bone. -

1 JOURNAL of VERTEBRATE PALEONTOLOGY a New
JOURNAL OF VERTEBRATE PALEONTOLOGY A new caenagnathid (Dinosauria: Oviraptorosauria) from the Horseshoe Canyon Formation of Alberta, Canada, and a reevaluation of the relationships of Caenagnathidae GREGORY F. FUNSTON*,,PHILIP J. CURRIE Department of Biological Sciences, CW 405, Biological Sciences Building, University of Alberta,Edmonton, Alberta, Canada T6G 2E9 [email protected]; [email protected] SUPPLEMENTARY DATA 1 1 CHARACTERS MODIFIED FROM LAMANNA ET AL. (2014) 78. Dentary: (0) elongate; (1) proportionally short and deep, with maximum depth of dentary between 25% and 50% of dentary length (with length measured from the tip of the jaw to the end of the posterodorsal process); (2) extremely short and deep, with maximum depth 50% or more of dentary length. [ORDERED] Modification—Removed [ORDERED] Justification—Mandibular variation through ontogeny in has not been qualified in oviraptorosaurs, nor has the degree of intraspecific variation. This character in particular is correlated with size in caenagnathids, such that larger specimens tend show state 0, and smaller specimens tend to show state 2, with a smooth gradient between. 84. Anterodorsal margin of dentary in lateral view: (0) straight; (1) concave; (2) broadly concave. [ORDERED] Modification—Removed [ORDERED] Justification—As above, though the opposite correlation to size is shown: large specimens tend to show state 2, and small specimens tend to show state 0. 176. Manual phalanx II-2: (0) longer than II-1; (1) subequal to or slightly shorter than II- 1; (2) distinctly shorter than II-1. [ORDERED] Modification—Removed [ORDERED] Justification—Caenagnathid manual proportions are highly variable, with a number of apparent reversals within clades. For example, Hagryphus giganteus, scored as character state 1 for this character, is consistently recovered as a basal caenagnathid, but within more derived caenagnathids, all three character states for this character are present, indicating that the character state can move both directions. -

Colour De Verre Molds: Feather
REUSABLE MOLDS FOR GLASS CASTING With either product, clean the See our website’s Learn section for mold with a stiff nylon brush and/ more instructions about priming or toothbrush to remove any old Colour de Verre molds with ZYP. kiln wash or boron nitride. (This step can be skipped if the mold is Filling the Feather brand new.) The suggested fill weight for the Feather is 330 to 340 grams. The If you are using Hotline Primo most simple way to fill the Feather Primer, mix the product according mold is to weigh out 330 of fine to directions. Apply the Primo frit and to evenly distribute the frit Primer™ with a soft artist’s brush in the mold. Fire the mold and frit (not a hake brush) and use a hair according the Casting Schedule Feather dryer to completely dry the coat. below. This design is also a perfect Create feathers that are as fanci- Give the mold four to five thin, candidate for our Wafer-Thin ful or realistic as you like with even coats drying each coat with a technique. One can read more Colour de Verre’s Feather de- hair dryer before applying the about this at www.colourdeverre.- sign. Once feathers are cast, next. Make sure to keep the Primo they can be slumped into amaz- com/go/wafer. ing decorative or functional well stirred as it settles quickly. pieces. The mold should be totally dry before filling. There is no reason to nnn pre-fire the mold. To use ZYP, hold the can 10 to 12 Feathers are a design element that inches from the mold. -

Saitta, ET, Gelernter, R., & Vinther, J
Saitta, E. T., Gelernter, R., & Vinther, J. (2018). Additional information on the primitive contour and wing feathering of paravian dinosaurs. Palaeontology, 61(2), 273-288. https://doi.org/10.1111/pala.12342 Peer reviewed version Link to published version (if available): 10.1111/pala.12342 Link to publication record in Explore Bristol Research PDF-document This is the author accepted manuscript (AAM). The final published version (version of record) is available online via Wiley at http://onlinelibrary.wiley.com/doi/10.1111/pala.12342/abstract . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Additional information on the primitive contour and wing feathering of paravian dinosaurs Evan T. Saitta1, Rebecca Gelernter2, & Jakob Vinther1,3 1School of Earth Sciences, University of Bristol, Bristol, United Kingdom; e-mails: [email protected] (ORCiD ID: orcid.org/0000-0002-9306-9060), [email protected] (ORCiD ID: orcid.org/0000-0002-3584-9616) 2Near Bird Studios, New Haven, Connecticut, USA; e-mail: [email protected] 3School of Biological Sciences, University of Bristol, Bristol, United Kingdom ABSTRACT: Identifying feather morphology in extinct dinosaurs is challenging due to dense overlapping of filaments within fossilized plumage and the fact that some extinct feather morphologies are unlike those seen in extant birds or those predicted from an ‘evo-devo’ model of feather evolution. -

Re-Evaluation of the Haarlem Archaeopteryx and the Radiation of Maniraptoran Theropod Dinosaurs Christian Foth1,3 and Oliver W
Foth and Rauhut BMC Evolutionary Biology (2017) 17:236 DOI 10.1186/s12862-017-1076-y RESEARCH ARTICLE Open Access Re-evaluation of the Haarlem Archaeopteryx and the radiation of maniraptoran theropod dinosaurs Christian Foth1,3 and Oliver W. M. Rauhut2* Abstract Background: Archaeopteryx is an iconic fossil that has long been pivotal for our understanding of the origin of birds. Remains of this important taxon have only been found in the Late Jurassic lithographic limestones of Bavaria, Germany. Twelve skeletal specimens are reported so far. Archaeopteryx was long the only pre-Cretaceous paravian theropod known, but recent discoveries from the Tiaojishan Formation, China, yielded a remarkable diversity of this clade, including the possibly oldest and most basal known clade of avialan, here named Anchiornithidae. However, Archaeopteryx remains the only Jurassic paravian theropod based on diagnostic material reported outside China. Results: Re-examination of the incomplete Haarlem Archaeopteryx specimen did not find any diagnostic features of this genus. In contrast, the specimen markedly differs in proportions from other Archaeopteryx specimens and shares two distinct characters with anchiornithids. Phylogenetic analysis confirms it as the first anchiornithid recorded outside the Tiaojushan Formation of China, for which the new generic name Ostromia is proposed here. Conclusions: In combination with a biogeographic analysis of coelurosaurian theropods and palaeogeographic and stratigraphic data, our results indicate an explosive radiation of maniraptoran coelurosaurs probably in isolation in eastern Asia in the late Middle Jurassic and a rapid, at least Laurasian dispersal of the different subclades in the Late Jurassic. Small body size and, possibly, a multiple origin of flight capabilities enhanced dispersal capabilities of paravian theropods and might thus have been crucial for their evolutionary success.