Generate Metabolic Map Poster
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Annotation-1 Annotation-1
Annotation-1 Baseline Resuscitation Normal Saline Resuscitation PFP Shock Annotation-1 Aminoacids Arginine and proline metabolism Carnitine and fatty acid metabolsim Glutamate metabolism Glycerophospholipid biosynthesis Glycolysis and sugars GSH homeostasis GSH homeostasis/Glyoxlate Hexosamine Indole and Tryptophan Nucleotides Other Panthothenate metabolism Pentose Phosphate Pathway Serine biosynthesis and one-carbon metabolism Signaling Sulfur metabolism TCA cycle urea cycle relative row min row max Baseline_14 Baseline_16 Baseline_13 Baseline_15 Baseline_22 Baseline_2 Baseline_12 Baseline_3 Baseline_4 Baseline_9 Baseline_7 Baseline_8 Shock_13 Shock_12 Shock_15 Shock_22 Shock_14 Shock_16 Shock_2 Shock_3 Shock_7 Shock_4 Shock_8 Shock_9 Res_NS_14 Res_NS_13 Res_NS_16 Res_NS_12 Res_NS_22 Res_NS_15 Res_PFP_2 Res_PFP_3 Res_PFP_7 Res_PFP_4 Res_PFP_8 Res_PFP_9 Annotation-1 Annotation-1 Annotation Annotation-1 L-Arginine Aminoacids L-Isoleucine Aminoacids Leucine Aminoacids L-Cysteine Aminoacids L-Alanine Aminoacids L-Aspartate Aminoacids L-Glutamate Aminoacids L-Glutamine Aminoacids L-Histidine Aminoacids L-Lysine Aminoacids L-Methionine Aminoacids L-Tyrosine Aminoacids L-Asparagine Aminoacids L-Threonine Aminoacids L-Cystine Aminoacids L-Serine Aminoacids L-Proline Aminoacids L-Valine Aminoacids L-Tryptophan Aminoacids Glycine Aminoacids L-Kynurenine Aminoacids L-Phenylalanine Aminoacids CMP Nucleotides 6-Hydroxynicotinate Nucleotides 5-6-Dihydrouracil Nucleotides AMP Nucleotides dAMP Nucleotides GMP Nucleotides Guanine Nucleotides 2-5-Dihydroxypyridine -

Itraq-Based Quantitative Proteomics Analysis Reveals the Mechanism Underlying the Weakening of Carbon Metabolism in Chlorotic Tea Leaves
Article iTRAQ-Based Quantitative Proteomics Analysis Reveals the Mechanism Underlying the Weakening of Carbon Metabolism in Chlorotic Tea Leaves Fang Dong 1,2, Yuanzhi Shi 1,2, Meiya Liu 1,2, Kai Fan 1,2, Qunfeng Zhang 1,2,* and Jianyun Ruan 1,2 1 Tea Research Institute, Chinese Academy of Agricultural Sciences, Hangzhou 310008, China; [email protected] (F.D.); [email protected] (Y.S.); [email protected] (M.L.); [email protected] (K.F.); [email protected] (J.R.) 2 Key Laboratory for Plant Biology and Resource Application of Tea, the Ministry of Agriculture, Hangzhou 310008, China * Correspondence: [email protected]; Tel.: +86-571-8527-0665 Received: 7 November 2018; Accepted: 5 December 2018; Published: 7 December 2018 Abstract: To uncover mechanism of highly weakened carbon metabolism in chlorotic tea (Camellia sinensis) plants, iTRAQ (isobaric tags for relative and absolute quantification)-based proteomic analyses were employed to study the differences in protein expression profiles in chlorophyll-deficient and normal green leaves in the tea plant cultivar “Huangjinya”. A total of 2110 proteins were identified in “Huangjinya”, and 173 proteins showed differential accumulations between the chlorotic and normal green leaves. Of these, 19 proteins were correlated with RNA expression levels, based on integrated analyses of the transcriptome and proteome. Moreover, the results of our analysis of differentially expressed proteins suggested that primary carbon metabolism (i.e., carbohydrate synthesis and transport) was inhibited in chlorotic tea leaves. The differentially expressed genes and proteins combined with photosynthetic phenotypic data indicated that 4-coumarate-CoA ligase (4CL) showed a major effect on repressing flavonoid metabolism, and abnormal developmental chloroplast inhibited the accumulation of chlorophyll and flavonoids because few carbon skeletons were provided as a result of a weakened primary carbon metabolism. -

35 Disorders of Purine and Pyrimidine Metabolism
35 Disorders of Purine and Pyrimidine Metabolism Georges van den Berghe, M.- Françoise Vincent, Sandrine Marie 35.1 Inborn Errors of Purine Metabolism – 435 35.1.1 Phosphoribosyl Pyrophosphate Synthetase Superactivity – 435 35.1.2 Adenylosuccinase Deficiency – 436 35.1.3 AICA-Ribosiduria – 437 35.1.4 Muscle AMP Deaminase Deficiency – 437 35.1.5 Adenosine Deaminase Deficiency – 438 35.1.6 Adenosine Deaminase Superactivity – 439 35.1.7 Purine Nucleoside Phosphorylase Deficiency – 440 35.1.8 Xanthine Oxidase Deficiency – 440 35.1.9 Hypoxanthine-Guanine Phosphoribosyltransferase Deficiency – 441 35.1.10 Adenine Phosphoribosyltransferase Deficiency – 442 35.1.11 Deoxyguanosine Kinase Deficiency – 442 35.2 Inborn Errors of Pyrimidine Metabolism – 445 35.2.1 UMP Synthase Deficiency (Hereditary Orotic Aciduria) – 445 35.2.2 Dihydropyrimidine Dehydrogenase Deficiency – 445 35.2.3 Dihydropyrimidinase Deficiency – 446 35.2.4 Ureidopropionase Deficiency – 446 35.2.5 Pyrimidine 5’-Nucleotidase Deficiency – 446 35.2.6 Cytosolic 5’-Nucleotidase Superactivity – 447 35.2.7 Thymidine Phosphorylase Deficiency – 447 35.2.8 Thymidine Kinase Deficiency – 447 References – 447 434 Chapter 35 · Disorders of Purine and Pyrimidine Metabolism Purine Metabolism Purine nucleotides are essential cellular constituents 4 The catabolic pathway starts from GMP, IMP and which intervene in energy transfer, metabolic regula- AMP, and produces uric acid, a poorly soluble tion, and synthesis of DNA and RNA. Purine metabo- compound, which tends to crystallize once its lism can be divided into three pathways: plasma concentration surpasses 6.5–7 mg/dl (0.38– 4 The biosynthetic pathway, often termed de novo, 0.47 mmol/l). starts with the formation of phosphoribosyl pyro- 4 The salvage pathway utilizes the purine bases, gua- phosphate (PRPP) and leads to the synthesis of nine, hypoxanthine and adenine, which are pro- inosine monophosphate (IMP). -

Discovery and Protein Engineering of Baeyer-Villiger Monooxygenases
Discovery and Protein Engineering of Baeyer-Villiger monooxygenases Inauguraldissertation zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Ernst-Moritz-Arndt-Universität Greifswald vorgelegt von Andy Beier geboren am 11.10.1988 in Parchim Greifswald, den 02.08.2017 I Dekan: Prof. Dr. Werner Weitschies 1. Gutachter: Prof. Dr. Uwe T. Bornscheuer 2. Gutachter: Prof. Dr. Marko Mihovilovic Tag der Promotion: 24.10.2017 II We need to learn to want what we have, not to have what we want, in order to get stable and steady happiness. - The Dalai Lama - III List of abbreviations % Percent MPS Methyl phenyl sulfide % (v/v) % volume per volume MPSO Methyl phenyl sulfoxide % (w/v) % weight per volume MPSO2 Methyl phenyl sulfone °C Degrees Celsius MTS Methyl p-tolyl sulfide µM µmol/L MTSO Methyl p-tolyl sulfoxide aa Amino acids MTSO2 Methyl p-tolyl sulfone + AGE Agarose gel electrophoresis NAD Nicotinamide adenine dinucleotide, oxidized aq. dest. Distilled water NADH Nicotinamide adenine dinucleotide, reduced + BLAST Basic Local Alignment Search NADP Nicotinamide adenine dinucleotide Tool phosphate, oxidized bp Base pair(s) NADPH Nicotinamide adenine dinucleotide phosphate, reduced BVMO Baeyer-Villiger monooxyge- OD600 Optical density at 600 nm nase CHMO Cyclohexanone monooxyge- PAGE Polyacrylamide gel electrophoresis nase Da Dalton PAMO Phenylacetone monooxygenase DMF Dimethyl formamide PCR Polymerase chain reaction DMSO Dimethyl sulfoxide PDB Protein Data Bank DMSO2 Dimethyl sulfone rpm Revolutions per minute DNA Desoxyribonucleic acid rv Reverse dNTP Desoxynucleoside triphosphate SDS Sodium dodecyl sulfate E. coli Escherichia coli SOC Super Optimal broth with Catabolite repression ee Enantiomeric excess TAE TRIS-Acetate-EDTA FAD Flavin adenine dinucleotide TB Terrific broth Fig. -

Product Profiles of Egyptian Henbane Premnaspirodiene
The Journal of Antibiotics (2016) 69, 524–533 & 2016 Japan Antibiotics Research Association All rights reserved 0021-8820/16 www.nature.com/ja ORIGINAL ARTICLE Biosynthetic potential of sesquiterpene synthases: product profiles of Egyptian Henbane premnaspirodiene synthase and related mutants Hyun Jo Koo1,3, Christopher R Vickery1,2,3,YiXu1, Gordon V Louie1, Paul E O'Maille1, Marianne Bowman1, Charisse M Nartey1, Michael D Burkart2 and Joseph P Noel1 The plant terpene synthase (TPS) family is responsible for the biosynthesis of a variety of terpenoid natural products possessing diverse biological functions. TPSs catalyze the ionization and, most commonly, rearrangement and cyclization of prenyl diphosphate substrates, forming linear and cyclic hydrocarbons. Moreover, a single TPS often produces several minor products in addition to a dominant product. We characterized the catalytic profiles of Hyoscyamus muticus premnaspirodiene synthase (HPS) and compared it with the profile of a closely related TPS, Nicotiana tabacum 5-epi-aristolochene synthase (TEAS). The profiles of two previously studied HPS and TEAS mutants, each containing nine interconverting mutations, dubbed HPS-M9 and TEAS- M9, were also characterized. All four TPSs were compared under varying temperature and pH conditions. In addition, we solved the X-ray crystal structures of TEAS and a TEAS quadruple mutant complexed with substrate and products to gain insight into the enzymatic features modulating product formation. These informative structures, along with product profiles, -

Photosynthetic Pigments in Diatoms
Mar. Drugs 2015, 13, 5847-5881; doi:10.3390/md13095847 OPEN ACCESS marine drugs ISSN 1660-3397 www.mdpi.com/journal/marinedrugs Review Photosynthetic Pigments in Diatoms Paulina Kuczynska 1, Malgorzata Jemiola-Rzeminska 1,2 and Kazimierz Strzalka 1,2,* 1 Faculty of Biochemistry, Biophysics and Biotechnology, Department of Plant Physiology and Biochemistry, Jagiellonian University, Gronostajowa 7, Krakow 30-387, Poland; E-Mails: [email protected] (P.K.); [email protected] (M.J.-R.) 2 Małopolska Centre of Biotechnology, Gronostajowa 7A, Krakow 30-387, Poland * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +48-126-646-509; Fax: +48-126-646-902. Academic Editor: Véronique Martin-Jézéquel Received: 10 July 2015 / Accepted: 7 September 2015 / Published: 16 September 2015 Abstract: Photosynthetic pigments are bioactive compounds of great importance for the food, cosmetic, and pharmaceutical industries. They are not only responsible for capturing solar energy to carry out photosynthesis, but also play a role in photoprotective processes and display antioxidant activity, all of which contribute to effective biomass and oxygen production. Diatoms are organisms of a distinct pigment composition, substantially different from that present in plants. Apart from light-harvesting pigments such as chlorophyll a, chlorophyll c, and fucoxanthin, there is a group of photoprotective carotenoids which includes β-carotene and the xanthophylls, diatoxanthin, diadinoxanthin, violaxanthin, antheraxanthin, and zeaxanthin, which are engaged in the xanthophyll cycle. Additionally, some intermediate products of biosynthetic pathways have been identified in diatoms as well as unusual pigments, e.g., marennine. Marine algae have become widely recognized as a source of unique bioactive compounds for potential industrial, pharmaceutical, and medical applications. -

Structure of Pigment Metabolic Pathways and Their Contributions to White Tepal Color Formation of Chinese Narcissus Tazetta Var
International Journal of Molecular Sciences Article Structure of Pigment Metabolic Pathways and Their Contributions to White Tepal Color Formation of Chinese Narcissus tazetta var. chinensis cv Jinzhanyintai Yujun Ren † ID , Jingwen Yang †, Bingguo Lu, Yaping Jiang, Haiyang Chen, Yuwei Hong, Binghua Wu and Ying Miao * Center for Molecular Cell and Systems Biology, Fujian Provincial Key Laboratory of Haixia Applied Plant Systems Biology, College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou 350002, China; [email protected] (Y.R.); [email protected] (J.Y.); [email protected] (B.L.); [email protected] (Y.J.); [email protected] (H.C.); [email protected] (Y.H.); [email protected] (B.W.) * Correspondence: [email protected]; Tel.:/Fax: +86-591-8639-2987 † These authors contributed equally to this work. Received: 7 August 2017; Accepted: 4 September 2017; Published: 8 September 2017 Abstract: Chinese narcissus (Narcissus tazetta var. chinensis) is one of the ten traditional flowers in China and a famous bulb flower in the world flower market. However, only white color tepals are formed in mature flowers of the cultivated varieties, which constrains their applicable occasions. Unfortunately, for lack of genome information of narcissus species, the explanation of tepal color formation of Chinese narcissus is still not clear. Concerning no genome information, the application of transcriptome profile to dissect biological phenomena in plants was reported to be effective. As known, pigments are metabolites of related metabolic pathways, which dominantly decide flower color. In this study, transcriptome profile and pigment metabolite analysis methods were used in the most widely cultivated Chinese narcissus “Jinzhanyintai” to discover the structure of pigment metabolic pathways and their contributions to white tepal color formation during flower development and pigmentation processes. -

(12) United States Patent (10) Patent No.: US 9,725,399 B2 Petrie Et Al
USO09725399B2 (12) United States Patent (10) Patent No.: US 9,725,399 B2 Petrie et al. (45) Date of Patent: Aug. 8, 2017 (54) LPID COMPRISING LONG CHAN (51) Int. Cl. POLYUNSATURATED FATTY ACDS C07C 69/587 (2006.01) CIIB I/O (2006.01) (71) Applicants: Commonwealth Scientific and (Continued) Industrial Research Organisation, (52) U.S. Cl. Acton, Australian Capital Territory CPC .............. C07C 69/587 (2013.01); A23D 9/00 (AU): Nuseed Pty Ltd, Laverton North, (2013.01); A61K 36/31 (2013.01): CIIB I/10 Victoria (AU); Grains Research and (2013.01); A61 K 2.236/00 (2013.01) Development Corporation, Barton, (58) Field of Classification Search Australian Capital Territory (AU) CPC .......................... C12N 15/8247; CO7C 69/587 See application file for complete search history. (72) Inventors: James Robertson Petrie, Goulburn (AU); Surinder Pal Singh, Downer (56) References Cited (AU); Pushkar Shrestha, Lawson U.S. PATENT DOCUMENTS (AU); Jason Timothy McAllister, Portarlington (AU); Robert Charles De 4,399.216 A 8, 1983 Axel et al. Feyter, Monash (AU); Malcolm David 5,004,863. A 4, 1991 Umbeck Devine, Vernon (CA) (Continued) (73) Assignees: COMMONWEALTH SCIENTIFIC FOREIGN PATENT DOCUMENTS AND INDUSTRIAL RESEARCH AU 667939 1, 1994 ORGANISATION, Campbell (AU): AU 200059710 B2 12/2000 NUSEED PTY LTD, Laverton North (Continued) (AU); GRAINS RESEARCH AND DEVELOPMENT CORPORATION, Barton (AU) OTHER PUBLICATIONS Ruiz-Lopez, N. et al., “Metabolic engineering of the omega-3 long (*) Notice: Subject to any disclaimer, the term of this chain polyunsaturated fatty acid biosynthetic pathway into trans patent is extended or adjusted under 35 genic plants' Journal of Experimental botany, 2012, vol. -

Relating Metatranscriptomic Profiles to the Micropollutant
1 Relating Metatranscriptomic Profiles to the 2 Micropollutant Biotransformation Potential of 3 Complex Microbial Communities 4 5 Supporting Information 6 7 Stefan Achermann,1,2 Cresten B. Mansfeldt,1 Marcel Müller,1,3 David R. Johnson,1 Kathrin 8 Fenner*,1,2,4 9 1Eawag, Swiss Federal Institute of Aquatic Science and Technology, 8600 Dübendorf, 10 Switzerland. 2Institute of Biogeochemistry and Pollutant Dynamics, ETH Zürich, 8092 11 Zürich, Switzerland. 3Institute of Atmospheric and Climate Science, ETH Zürich, 8092 12 Zürich, Switzerland. 4Department of Chemistry, University of Zürich, 8057 Zürich, 13 Switzerland. 14 *Corresponding author (email: [email protected] ) 15 S.A and C.B.M contributed equally to this work. 16 17 18 19 20 21 This supporting information (SI) is organized in 4 sections (S1-S4) with a total of 10 pages and 22 comprises 7 figures (Figure S1-S7) and 4 tables (Table S1-S4). 23 24 25 S1 26 S1 Data normalization 27 28 29 30 Figure S1. Relative fractions of gene transcripts originating from eukaryotes and bacteria. 31 32 33 Table S1. Relative standard deviation (RSD) for commonly used reference genes across all 34 samples (n=12). EC number mean fraction bacteria (%) RSD (%) RSD bacteria (%) RSD eukaryotes (%) 2.7.7.6 (RNAP) 80 16 6 nda 5.99.1.2 (DNA topoisomerase) 90 11 9 nda 5.99.1.3 (DNA gyrase) 92 16 10 nda 1.2.1.12 (GAPDH) 37 39 6 32 35 and indicates not determined. 36 37 38 39 S2 40 S2 Nitrile hydration 41 42 43 44 Figure S2: Pearson correlation coefficients r for rate constants of bromoxynil and acetamiprid with 45 gene transcripts of ECs describing nucleophilic reactions of water with nitriles. -

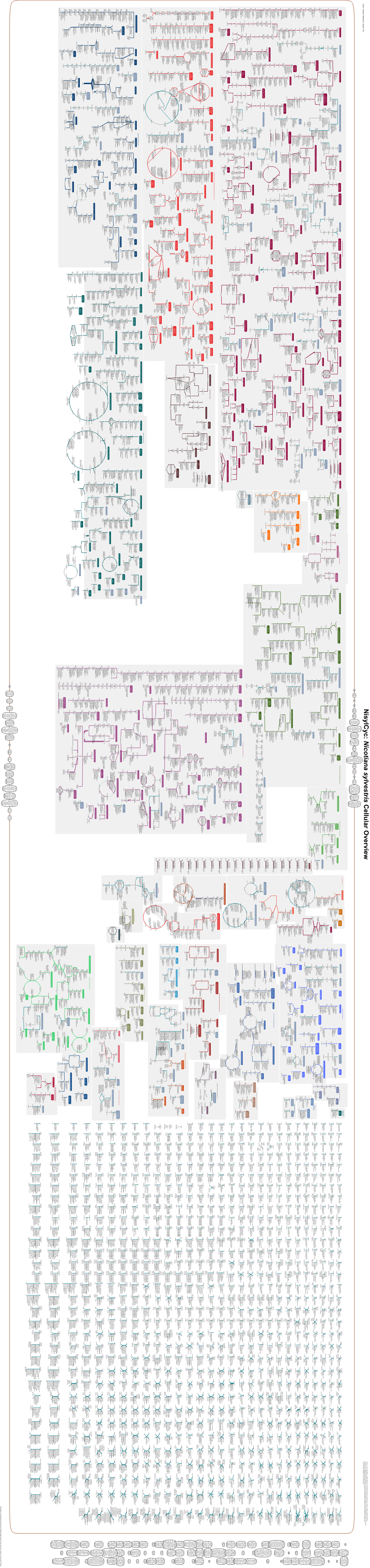
Generated by SRI International Pathway Tools Version 25.0, Authors S
Authors: Pallavi Subhraveti Ron Caspi Quang Ong Peter D Karp An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Ingrid Keseler Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. Gcf_000725805Cyc: Streptomyces xanthophaeus Cellular Overview Connections between pathways are omitted for legibility. -

1 AMINO ACIDS Commonly, 21 L-Amino Acids Encoded by DNA Represent the Building Blocks of Animal, Plant, and Microbial Proteins
1 AMINO ACIDS Commonly, 21 L-amino acids encoded by DNA represent the building blocks of animal, plant, and microbial proteins. The basic amino acids encountered in proteins are called proteinogenic amino acids 1.1). Biosynthesis of some of these amino acids proceeds by ribosomal processes only in microorganisms and plants and the ability to synthesize them is lacking in animals, including human beings. These amino acids have to be obtained in the diet (or produced by hydrolysis of body proteins) since they are required for normal good health and are referred to as essential amino acids. The essential amino acids are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. The rest of encoded amino acids are referred to as non-essential amino acids (alanine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine). Arginine and histidine are classified as essential, sometimes as semi-essential amino acids, as their amount synthesized in the body is not sufficient for normal growth of children. Although it is itself non-essential, cysteine (classified as conditionally essential amino acid) can partly replace methionine, which is an essential amino acid. Similarly, tyrosine can partly replace phenylalanine. 1.1 The glutamic acid group 1.1.1 Glutamic acid and glutamine Free ammonium ions are toxic to living cells and are rapidly incorporated into organic compounds. One of such transformations is the reaction of ammonia with 2-oxoglutaric acid from the citric acid cycle to produce L-glutamic acid. This reaction is known as reductive amination. Glutamic acid is accordingly the amino acid generated first as both constituent of proteins and a biosynthetic precursor. -

(10) Patent No.: US 8119385 B2
US008119385B2 (12) United States Patent (10) Patent No.: US 8,119,385 B2 Mathur et al. (45) Date of Patent: Feb. 21, 2012 (54) NUCLEICACIDS AND PROTEINS AND (52) U.S. Cl. ........................................ 435/212:530/350 METHODS FOR MAKING AND USING THEMI (58) Field of Classification Search ........................ None (75) Inventors: Eric J. Mathur, San Diego, CA (US); See application file for complete search history. Cathy Chang, San Diego, CA (US) (56) References Cited (73) Assignee: BP Corporation North America Inc., Houston, TX (US) OTHER PUBLICATIONS c Mount, Bioinformatics, Cold Spring Harbor Press, Cold Spring Har (*) Notice: Subject to any disclaimer, the term of this bor New York, 2001, pp. 382-393.* patent is extended or adjusted under 35 Spencer et al., “Whole-Genome Sequence Variation among Multiple U.S.C. 154(b) by 689 days. Isolates of Pseudomonas aeruginosa” J. Bacteriol. (2003) 185: 1316 1325. (21) Appl. No.: 11/817,403 Database Sequence GenBank Accession No. BZ569932 Dec. 17. 1-1. 2002. (22) PCT Fled: Mar. 3, 2006 Omiecinski et al., “Epoxide Hydrolase-Polymorphism and role in (86). PCT No.: PCT/US2OO6/OOT642 toxicology” Toxicol. Lett. (2000) 1.12: 365-370. S371 (c)(1), * cited by examiner (2), (4) Date: May 7, 2008 Primary Examiner — James Martinell (87) PCT Pub. No.: WO2006/096527 (74) Attorney, Agent, or Firm — Kalim S. Fuzail PCT Pub. Date: Sep. 14, 2006 (57) ABSTRACT (65) Prior Publication Data The invention provides polypeptides, including enzymes, structural proteins and binding proteins, polynucleotides US 201O/OO11456A1 Jan. 14, 2010 encoding these polypeptides, and methods of making and using these polynucleotides and polypeptides.