Deeply Conserved Synteny Resolves Early Events in Vertebrate Evolution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
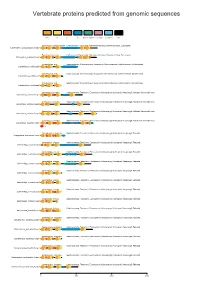
Vertebrate Proteins Predicted from Genomic Sequences
Vertebrate proteins predicted from genomic sequences VWD C8 TIL PTS Mucin2_WxxW F5_F8_type_C FCGBP_N VWC Lethenteron_camtschaticum Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Lethenteron Lethenteron_camtschaticum.0.pep1 Petromyzon_marinus Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Petromyzon Petromyzon_marinus.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep2 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep1 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep2 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.1.pep1 TILa Cynoglossus_semilaevis Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Teleostei; Cynoglossus_semilaevis.1.pep1 -

Copyrighted Material
Index INDEX Note: page numbers in italics refer to fi gures, those in bold refer to tables and boxes. abducens nerve 55 activity cycles 499–522 inhibition 485 absorption effi ciency 72 annual patterns 515, 516, 517–22 interactions 485–6 abyssal zone 393 circadian rhythms 505 prey 445 Acanthaster planci (Crown-of-Thorns Starfi sh) diel patterns 499, 500, 501–2, 503–4, reduction 484 579 504–7 aggressive mimicry 428, 432–3 Acanthocybium (Wahoo) 15 light-induced 499, 500, 501–2, 503–4, aggressive resemblance 425–6 Acanthodii 178, 179 505 aglomerular 52 Acanthomorpha 284–8, 289 lunar patterns 507–9 agnathans Acanthopterygii 291–325 seasonal 509–15 gills 59, 60 Atherinomorpha 293–6 semilunar patterns 507–9 osmoregulation 101, 102 characteristics 291–2 supra-annual patterns 515, 516, 517–22 phylogeny 202 distribution 349, 350 tidal patterns 506–7 ventilation 59, 60 jaws 291 see also migration see also hagfi shes; lampreys Mugilomorpha 292–3, 294 adaptive response 106 agnathous fi shes see jawless fi shes pelagic 405 adaptive zones 534 agonistic interactions 83–4, 485–8 Percomorpha 296–325 adenohypophysis 91, 92 chemically mediated 484 pharyngeal jaws 291 adenosine triphosphate (ATP) 57 sound production 461–2 phylogeny 292, 293, 294 adipose fi n 35 visual 479 spines 449, 450 adrenocorticotropic hormone (ACTH) 92 agricultural chemicals 605 Acanthothoraciformes 177 adrianichthyids 295 air breathing 60, 61–2, 62–4 acanthurids 318–19 adult fi shes 153, 154, 155–7 ammonia production 64, 100–1 Acanthuroidei 12, 318–19 death 156–7 amphibious 60 Acanthurus bahianus -

2R Or Not 2R Is Not the Question Anymore
CORRESPONDENCE LINK TO ORIGINAL ARTICLE LINK TO INITIAL CORRESPONDENCE origin of evolutionary novelties are highly interesting questions, but they remain 2R or not 2R is not the largely unsolved11. However, evidence for the two rounds of genome duplication dur- question anymore ing chordate evolution is very strong, and it would seem safe to say that the debate Yves Van de Peer, Steven Maere and Axel Meyer over 2R is settled and is no longer an open question12. As we point out in our Opinion article1, it is only the evolutionary effects of In his comments on our Opinion article in passing in our recent review on WGDs events such as WGDs on evolution that are (The evolutionary significance of ancient and their significance for evolution in Nature debated1,11, and no longer whether or not genome duplications. Nature Rev. Genet. Reviews Genetics1. two rounds of WGD occurred during the 10, 725–732 (2009))1 Amir Ali Abbasi Abbasi2, however, questions the support evolution of chordates. (Piecemeal or big bangs: correlating the for the 2R hypothesis, claiming that it still Yves Van de Peer and Steven Maere are at the vertebrate evolution with proposed models remains debated today. He points out cor- Department of Plant Systems Biology, VIB (Flanders of gene expansion events. Nature Rev. Genet. rectly that evidence for the 2R hypothesis Institute of Biotechnology), B-9052 Ghent, Belgium. 6 Jan 2010 (doi:10.1038/nrg2600-c1))2 was based initially on data from only a Axel Meyer is at the Department of Biology, University argues that it is not justified to speculate small number of genes and vertebrate and of Konstanz, D-78457 Konstanz, Germany. -

Epithelial Sodium Channel (Enac) Family: Phylogeny, Structure–Function, Tissue Distribution, and Associated Inherited Diseases
Gene 579 (2016) 95–132 Contents lists available at ScienceDirect Gene journal homepage: www.elsevier.com/locate/gene Gene wiki review Epithelial sodium channel (ENaC) family: Phylogeny, structure–function, tissue distribution, and associated inherited diseases Israel Hanukoglu a,⁎, Aaron Hanukoglu b,c a Laboratory of Cell Biology, Faculty of Natural Sciences, Ariel University, Ariel, Israel b Division of Pediatric Endocrinology, E. Wolfson Medical Center, Holon, Israel c Sackler School of Medicine, Tel-Aviv University, Tel Aviv, Israel article info abstract Article history: The epithelial sodium channel (ENaC) is composed of three homologous subunits and allows the flow of Na+ ions Received 7 September 2015 across high resistance epithelia, maintaining body salt and water homeostasis. ENaC dependent reabsorption of Received in revised form 20 December 2015 Na+ in the kidney tubules regulates extracellular fluid (ECF) volume and blood pressure by modulating osmolar- Accepted 22 December 2015 ity. In multi-ciliated cells, ENaC is located in cilia and plays an essential role in the regulation of epithelial surface Available online 7 January 2016 liquid volume necessary for cilial transport of mucus and gametes in the respiratory and reproductive tracts respectively. Keywords: Ion channels The subunits that form ENaC (named as alpha, beta, gamma and delta, encoded by genes SCNN1A, SCNN1B, Epithelia SCNN1G, and SCNN1D) are members of the ENaC/Degenerin superfamily. The earliest appearance of ENaC Evolution orthologs is in the genomes of the most ancient vertebrate taxon, Cyclostomata (jawless vertebrates) including Transmembrane proteins lampreys, followed by earliest representatives of Gnathostomata (jawed vertebrates) including cartilaginous Kidney sharks. Among Euteleostomi (bony vertebrates), Actinopterygii (ray finned-fishes) branch has lost ENaC genes. -

Timing and Mechanism of Ancient Vertebrate Genome Duplications – the Adventure of a Hypothesis
ARTICLE IN PRESS TIGS 365 Review TRENDS in Genetics Vol.xx No.xx Monthxxxx Timing and mechanism of ancient vertebrate genome duplications – the adventure of a hypothesis Georgia Panopoulou and Albert J. Poustka Evolution and Development Group, Department of Vertebrate Genomics, Max-Planck Institut fu¨ r Molekulare Genetik, Ihnestrasse 73, D-14195 Berlin, Germany Complete genome doubling has long-term conse- period following the split of the cephalochordate and quences for the genome structure and the subsequent vertebrate lineages and before the emergence of gnathos- evolution of an organism. It has been suggested that tomes (Figure 1). Based on the apparent stepwise increase two genome duplications occurred at the origin of in the gene copy-number from invertebrates to jawless vertebrates (known as the 2R hypothesis). However, there has been considerable debate as to whether these were two successive duplications, or whether a single Glossary duplication occurred, followed by large-scale segmental (AB)(CD) topology measure: the nodes of the phylogenetic tree of four duplications. In this article, we review and compare the duplicates generated from two duplication events should have the (AB)(CD) evidence for the 2R duplications from vertebrate genomes topology where the dates of duplication for the (AB) and (CD) nodes are the same. Neighbor genes within paralogons that have the same topology are with similar data from other more recent polyploids. assumed to have been generated through the same event. Agnathans: jawless vertebrates. Aneuploidy: the loss or addition of one or more specific chromosomes to the normal set of chromosomes of an organism (e.g. a form of aneuploidy is Introduction trisomy 21). -

“Parent-Daughter” Relationships Among Vertebrate Paralogs
Reconstruction of the deep history of “Parent-Daughter” relationships among vertebrate paralogs Haiming Tang*, Angela Wilkins Mercury Data Science, Houston, TX, 77098 * Corresponding author Abstract: Gene duplication is a major mechanism through which new genetic material is generated. Although numerous methods have been developed to differentiate the ortholog and paralogs, very few differentiate the “Parent-Daughter” relationship among paralogous pairs. As coined by the Mira et al, we refer the “Parent” copy as the paralogous copy that stays at the original genomic position of the “original copy” before the duplication event, while the “Daughter” copy occupies a new genomic locus. Here we present a novel method which combines the phylogenetic reconstruction of duplications at different evolutionary periods and the synteny evidence collected from the preserved homologous gene orders. We reconstructed for the first time a deep evolutionary history of “Parent-Daughter” relationships among genes that were descendants from 2 rounds of whole genome duplications (2R WGDs) at early vertebrates and were further duplicated in later ceancestors like early Mammalia and early Primates. Our analysis reveals that the “Parent” copy has significantly fewer accumulated mutations compared with the “Daughter” copy since their divergence after the duplication event. More strikingly, we found that the “Parent” copy in a duplication event continues to be the “Parent” of the younger successive duplication events which lead to “grand-daughters”. Data availability: we have made the “Parent-Daughter” relationships publicly available at https://github.com/haimingt/Parent-Daughter-In-Paralogs/ Introduction Gene duplication has been widely accepted as a shaping force in evolution (Zhang, et al. -

NIH Public Access Author Manuscript Dev Dyn
NIH Public Access Author Manuscript Dev Dyn. Author manuscript; available in PMC 2010 June 25. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Dev Dyn. 2009 June ; 238(6): 1249±1270. doi:10.1002/dvdy.21891. Comparative and Developmental Study of the Immune System in Xenopus Jacques Robert1,* and Yuko Ohta2 1Department of Microbiology and Immunology, University of Rochester Medical Center, Rochester, New York 2Department of Microbiology and Immunology, University of Maryland School of Medicine, Baltimore, Maryland Abstract Xenopus laevis is the model of choice for evolutionary, comparative, and developmental studies of immunity, and invaluable research tools including MHC-defined clones, inbred strains, cell lines, and monoclonal antibodies are available for these studies. Recent efforts to use Silurana (Xenopus) tropicalis for genetic analyses have led to the sequencing of the whole genome. Ongoing genome mapping and mutagenesis studies will provide a new dimension to the study of immunity. Here we review what is known about the immune system of X. laevis integrated with available genomic information from S. tropicalis. This review provides compelling evidence for the high degree of similarity and evolutionary conservation between Xenopus and mammalian immune systems. We propose to build a powerful and innovative comparative biomedical model based on modern genetic technologies that takes take advantage of X. laevis and S. tropicalis, as well as the whole Xenopus genus. Keywords comparative immunology; developmental immunology; evolution of immunity; genomics INTRODUCTION From an evolutionary point of view, Xenopus is one “connecting” taxon that links mammals to vertebrates of more ancient origin (bony and cartilaginous fishes), that shared a common ancestor ~350 MYA (Pough et al., 2002). -

Of Genome Duplications and the Evolution of the Glycolytic Pathway in Vertebrates Dirk Steinke†1,3, Simone Hoegg†1, Henner Brinkmann2 and Axel Meyer*1
BMC Biology BioMed Central Research article Open Access Three rounds (1R/2R/3R) of genome duplications and the evolution of the glycolytic pathway in vertebrates Dirk Steinke†1,3, Simone Hoegg†1, Henner Brinkmann2 and Axel Meyer*1 Address: 1Lehrstuhl für Evolutionsbiologie und Zoologie, Department of Biology, University of Konstanz, 78457 Konstanz, Germany, 2Département de biochimie. Université de Montreal, Montreal, QC, H3C3J7, Canada and 3Canadian Centre for DNA Barcoding, Biodiversity Institute of Ontario, University of Guelph, Guelph, ON, N1G 2W1, Canada Email: Dirk Steinke - [email protected]; Simone Hoegg - [email protected]; Henner Brinkmann - [email protected]; Axel Meyer* - [email protected] * Corresponding author †Equal contributors Published: 06 June 2006 Received: 03 February 2006 Accepted: 06 June 2006 BMC Biology 2006, 4:16 doi:10.1186/1741-7007-4-16 This article is available from: http://www.biomedcentral.com/1741-7007/4/16 © 2006 Steinke et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Evolution of the deuterostome lineage was accompanied by an increase in systematic complexity especially with regard to highly specialized tissues and organs. Based on the observation of an increased number of paralogous genes in vertebrates compared with invertebrates, two entire genome duplications (2R) were proposed during the early evolution of vertebrates. Most glycolytic enzymes occur as several copies in vertebrate genomes, which are specifically expressed in certain tissues. -

“Primordial Immune Complex”: Origins of MHC Class I and Antigen Receptors Revealed by Comparative Genomics
Inferring the ''Primordial Immune Complex'': Origins of MHC Class I and Antigen Receptors Revealed by Comparative Genomics This information is current as of September 29, 2021. Yuko Ohta, Masanori Kasahara, Timothy D. O'Connor and Martin F. Flajnik J Immunol published online 6 September 2019 http://www.jimmunol.org/content/early/2019/09/05/jimmun ol.1900597 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2019/09/06/jimmunol.190059 Material 7.DCSupplemental http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 29, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2019 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published September 6, 2019, doi:10.4049/jimmunol.1900597 The Journal of Immunology Inferring the “Primordial Immune Complex”: Origins of MHC Class I and Antigen Receptors Revealed by Comparative Genomics Yuko Ohta,* Masanori Kasahara,† Timothy D. O’Connor,‡,x,{,‖ and Martin F. Flajnik* Comparative analyses suggest that the MHC was derived from a prevertebrate “primordial immune complex” (PIC). -

Bos Taurus, Cattle, Cow at Geochembio: Taxonomy, Brief Facts
http://www.GeoChemBio.com: Bos taurus, cattle, cow ● Taxonomy ● Brief facts ● Developmental stages ● Digestive system ● Diagram of digestive system ● Some Bovidae species ● Bovidae species ● Cattle domestication ● Cattle domestication diagram ● Photo gallery Taxonomy cellular organisms - Eukaryota - Fungi/Metazoa group - Metazoa - Eumetazoa - Bilateria - Coelomata - Deuterostomia - Chordata - Craniata - Vertebrata - Gnathostomata - Teleostomi - Euteleostomi - Sarcopterygii - Tetrapoda - Amniota - Mammalia - Theria - Eutheria - Laurasiatheria - Cetartiodactyla - Ruminantia - Pecora - Bovidae - Bovinae - Bos - Bos taurus The two principal taxonomic groups of domestic cattle are Bos taurus (taurine cattle) and Bos indicus (zebu cattle). General description ● Domestic cows are common throughout the world. They are grown and bred for food - meat and milk production, as well as for working - plowing and moving heavy loads. ● Domestic cows are social animals and live in herds which are structured according to a dominance hierarchy. ● Cows feed on grasses and other herbaceous plants. An average cow can consume about 70 kg of grass in an 8 hour day. Cows are ruminants. They have a four chambered stomach. Cow's milk allergy vs. cow's milk intolerance ● Cow's milk allergy and cow's milk intolerance are two different concepts that are used interchangeably. The former is an immunologically mediated reaction to the milk's contents, not unlike to any other allergic reactions, for example, to eggs, corn, nuts, etc. The latter is non-immunologic reaction to the cow's milk, the most common cause of which is deficiency of lactase - the enzyme that breaks down one of the main nutrients of the milk - lactose. Importance of bovine genome ● The bovine genome serves as a reference non-primate, non-rodent, eutherian genome. -

Multiple Large-Scale Gene and Genome Duplications During the Evolution of Hexapods
Multiple large-scale gene and genome duplications during the evolution of hexapods Zheng Lia,1, George P. Tileyb,c,1, Sally R. Galuskaa, Chris R. Reardona, Thomas I. Kiddera, Rebecca J. Rundella,d, and Michael S. Barkera,2 aDepartment of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721; bDepartment of Biology, University of Florida, Gainesville, FL 32611; cDepartment of Biology, Duke University, Durham, NC 27708; and dDepartment of Environmental and Forest Biology, State University of New York College of Environmental Science and Forestry, Syracuse, NY 13210 Edited by Michael Freeling, University of California, Berkeley, CA, and approved March 12, 2018 (received for review June 14, 2017) Polyploidy or whole genome duplication (WGD) is a major contrib- than 800,000 described hexapod species (25) are known polyploids utor to genome evolution and diversity. Although polyploidy is (17, 20). However, until recently there were limited data available recognized as an important component of plant evolution, it is to search for evidence of paleopolyploidy among the hexapods generally considered to play a relatively minor role in animal and other animal clades. Thus, the contributions of polyploidy to evolution. Ancient polyploidy is found in the ancestry of some animal evolution and the differences with plant evolution have animals, especially fishes, but there is little evidence for ancient remained unclear. WGDs in other metazoan lineages. Here we use recently published To search for evidence of WGDs among the hexapods, we transcriptomes and genomes from more than 150 species across the leveraged recently released genomic data for the insects (26). insect phylogeny to investigate whether ancient WGDs occurred Combined with additional datasets from public databases, we assembled 128 transcriptomes and 27 genomes with at least one during the evolution of Hexapoda, the most diverse clade of animals. -

Origin and Evolution of the Adaptive Immune System: Genetic Events and Selective Pressures
REVIEWS Origin and evolution of the adaptive immune system: genetic events and selective pressures Martin F. Flajnik* and Masanori Kasahara‡ Abstract | The adaptive immune system (AIS) in mammals, which is centred on lymphocytes bearing antigen receptors that are generated by somatic recombination, arose approximately 500 million years ago in jawed fish. This intricate defence system consists of many molecules, mechanisms and tissues that are not present in jawless vertebrates. Two macroevolutionary events are believed to have contributed to the genesis of the AIS: the emergence of the recombination-activating gene (RAG) transposon, and two rounds of whole-genome duplication. It has recently been discovered that a non-RAG-based AIS with similarities to the jawed vertebrate AIS — including two lymphoid cell lineages — arose in jawless fish by convergent evolution. We offer insights into the latest advances in this field and speculate on the selective pressures that led to the emergence and maintenance of the AIS. Somatic hypermutation The adaptive immune system (AIS) is fascinating mechanisms, but jawless fish (Agnathans) apparently Mutation of the variable gene to both scientists and laymen: we have a specific yet had none. This mystifying finding led to the ‘big bang’ after mature B cells are incredibly diverse system that can fight myriad patho‑ theory of AIS emergence6, which is one of the main stimulated. It results in affinity maturation of the antibody gens and has a ‘memory’ — the basis of vaccination topics of this Review. response. Like the class switch, — that enables a rapid response to previously encoun‑ The discovery in jawless fish of a lymphoid cell‑based it requires activation-induced tered pathogens.