Exploiting the Genetic Diversity of Wild Ancestors and Relatives of Wheat for Its Improvement Jagdeep Singh Sidhu South Dakota State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SRP472 3 Comparison of Rye and Triticale As Forages for Grazing
Selected Articles from 1985 Report of Agricultural Research Southeast Branch Experiment Station Agricultural Experiment Station Kansas State University 16 Comparison of Rye and Triticale as Forages for Grazing Stocker Cattle Winter annual small grains are frequently grazed during late fall and early spring in southeastern Kansas. Wheat is often the crop of choice, especially if grain production is the primary objective. If pasture is the main consideration, there are probably other small grains that will yield more forage and produce a greater quantity of beef cattle weight gain per acre than wheat. Research has been conducted at the Southeast Kansas Experiment Station to determine which winter annual small grains will result in maximum forage and beef production in a graze-out program. A study conducted in 1981-82 revealed that triticale produced nearly twice as much beef liveweight gain per acre as Newton wheat. Results from a 1982-83 study indicated that a mixture of 2/3 rye and 1/3 wheat produced over three times as much beef liveweight per acre as triticale. The following study was conducted to compare rye and triticale with respect to performance of grazing stocker cattle. Procedure: On September 19, 1983, two 5-acre fields were seeded with winter annuals. One field was seeded with 105 lb of triticale per acre and the other was seeded with 89 lb of Bonel rye per acre. At seeding time, 25-65-70 lb of N-P2O5-K2O per acre was applied and on November 14, 1984, 50 lb of N per acre was applied to each pasture. -

Cryptococcus Neoformans Can Form Titan-Like Cells in Vitro in Response to Multiple Signals
RESEARCH ARTICLE Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals Nuria Trevijano-Contador1¤, Haroldo Cesar de Oliveira1,2, RocõÂo GarcõÂa-Rodas1, SueÂlen Andreia Rossi1, Irene Llorente1, A ngel Zaballos3, Guilhem Janbon4, JoaquõÂn Ariño5, O scar Zaragoza1* 1 Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Majadahonda, Madrid, Spain, 2 Universidade Estadual Paulista (UNESP), Faculdade de Ciências Farmacêuticas, CaÃmpus Araraquara, Departamento de AnaÂlises ClõÂnicas, LaboratoÂrio de Micologia ClõÂnica, Araraquara, São Paulo, Brazil, 3 Genomics Unit, Core Scientific Services, Instituto de Salud Carlos III, a1111111111 Majadahonda, Madrid, Spain, 4 Institut Pasteur, Unite Biologie des ARN des Pathogènes Fongiques, a1111111111 DeÂpartement de Mycologie, Paris, France, 5 Institut de Biotecnologia i Biomedicina and Departament de a1111111111 BioquõÂmica i Biologia Molecular, Universitat Autònoma de Barcelona, Cerdanyola del Vallès, Spain a1111111111 a1111111111 ¤ Current address: Albert Einstein College of Medicine, Bronx, New York, United States of America. * [email protected] Abstract OPEN ACCESS Cryptococcus neoformans is an encapsulated pathogenic yeast that can change the size of Citation: Trevijano-Contador N, de Oliveira HC, GarcõÂa-Rodas R, Rossi SA, Llorente I, Zaballos AÂ, the cells during infection. In particular, this process can occur by enlarging the size of the et al. (2018) Cryptococcus neoformans can form capsule without modifying the size of the cell body, or by increasing the diameter of the cell titan-like cells in vitro in response to multiple body, which is normally accompanied by an increase of the capsule too. This last process signals. PLoS Pathog 14(5): e1007007. https://doi. leads to the formation of cells of an abnormal enlarged size denominated titan cells. -

John Percival
THE LINNEAN Wheat Taxonomy: the legacy of John Percival THE LINNEAN SOCIETY OF LONDON BURLINGTON HOUSE, PICCADILLY, LONDON WlJ OBF SPECIAL ISSUE No 3 2001 ACADEMIC PRESS LIMITED 32 Jam.estown Road London NWl 7BY Printed on acid free paper © 2001 The Linnean Society of London All rights reserved. No part of this book may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage or retrieval system without permission in writing from the publisher. The designations of geographic entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of the publishers, the Linnean Society, the editors or any other participating organisations concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of The Society, the editors, or other participating organisations. Printed in Great Britain. Wheat Taxonomy: the legacy of John Percival Conference Participants (most of whom are identified by number on the key to the group photograph above). I. M. Ambrose,; 2. J. Bingham, UK; 3. R. Blatter, Switzerland; 4. A. Bomer, Germany; 5. A. Brandolini Italy; 6. R. Brigden, UK; 7. A. H. Bunting, UK; 8. P. Caligari, UK; 9. E.M.L.P. Clauss, USA; 10. P.O. Clauss, USA; 11 . K. Clavel, France; 12. P. Davis, UK; 13. J. Dvohik, USA; 14. !. Faberova, Czech Republic; 15 . A. A. Filatenko, Russia; 16. -

Article on Genetic Markers for Bunt Resistance From
Let’s make grain great again Click here to sign up for the newsletter The Landrace Newsletter no. 5 May 2021 A new growing season is ahead of us, and I greet the spring with news from both future and past from the organic grain sector. I wish you joyfull reading Anders Borgen Content in this newsletter Open field day and general assembly in Landsorten, Tuesday 22. June..............................................2 But now then, is it Landsorten or Agrologica, selling organic seed in future?...................................2 Mobile stone mill for local production................................................................................................3 Nordic grain festival 28th-30th October 2021 in Norway.....................................................................4 News from Agrologica science lab......................................................................................................4 Genetic markers for bunt resistance - news from LIVESEED-, Økosort-II and bunt projects......4 Acid rain and gluten-index..............................................................................................................4 Zanduri, Macha, and the hailstorm in Georgia...............................................................................6 Colchic emmer (Triticum paleochochicum)...............................................................................6 Emmer........................................................................................................................................7 Durum........................................................................................................................................7 -

Molecular Genetic Characterization of Ptr Toxc-Tsc1 Interaction
MOLECULAR GENETIC CHARACTERIZATION OF PTR TOXC-TSC1 INTERACTION AND COMPARATIVE GENOMICS OF PYRENOPHORA TRITICI-REPENTIS A Dissertation Submitted to the Graduate Faculty of the North Dakota State University of Agriculture and Applied Science By Gayan Kanishka Kariyawasam In Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Department: Plant Pathology November 2018 Fargo, North Dakota North Dakota State University Graduate School Title MOLECULAR GENETIC CHARACTERIZATION OF PTR TOXC-TSC1 INTERACTION AND COMPARATIVE GENOMICS OF PYRENOPHORA TRITICI-REPENTIS By Gayan Kanishka Kariyawasam The Supervisory Committee certifies that this disquisition complies with North Dakota State University’s regulations and meets the accepted standards for the degree of DOCTOR OF PHILOSOPHY SUPERVISORY COMMITTEE: Dr. Zhaohui Liu Chair Dr. Shaobin Zhong Dr. Justin D. Faris Dr. Phillip E. McClean Dr. Timothy L. Friesen Approved: November 7, 2018 Jack Rasmussen Date Department Chair ABSTRACT Tan spot of wheat, caused by Pyrenophora tritici-repentis, is an economically important disease worldwide. The disease system is known to involve three pairs of interactions between fungal-produced necrotrophic effectors (NEs) and the wheat sensitivity genes, namely Ptr ToxA- Tsn1, Ptr ToxB-Tsc2 and Ptr ToxC-Tsc1, all of which result in susceptibility. Many lines of evidence also suggested the involvement of additional fungal virulence and host resistance factors. Due to the non-proteinaceous nature, Ptr ToxC, has not been purified and the fungal gene (s) controlling Ptr ToxC production is unknown. The objective for the first part of research is to map the fungal gene (s) controlling Ptr ToxC production. Therefore, A bi-parental fungal population segregating for Ptr ToxC production was first developed from genetically modified heterothallic strains of AR CrossB10 (Ptr ToxC producer) and 86-124 (Ptr ToxC non-producer), and then was genotyped and phenotyped. -
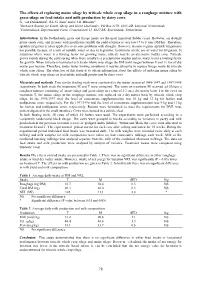
78 the Effects of Replacing Maize Silage by Triticale Whole Crop Silage
The effects of replacing maize silage by triticale whole crop silage in a roughage mixture with grass silage on feed intake and milk production by dairy cows G. van Duinkerken1, R.L.G. Zom1 and E.J.B. Bleumer2 1Research Station for Cattle, Sheep and Horse Husbandry, PO Box 2176, 8203 AD, Lelystad, Netherlands 2Cranendonck, Experimental Farm, Cranendonck 11, 6027 RK, Soerendonk, Netherlands Introduction In the Netherlands, grass and forage maize are the most important fodder crops. However, on drought prone sandy soils, and in years with insufficient rainfall the yield of maize is very low (7 to 8 tons DM/ha). Therefore, sprinkle irrigation is often applied to overcome problems with drought. However, in some regions sprinkle irrigation is not possible because of a lack of suitable water or due to legislative restrictions on the use of water for irrigation. In situations where water is a limiting factor for growing maize, triticale may be an alternative fodder crop. Triticale grows mainly during the early spring when there usually is a precipitation surplus and so, water is not a limiting factor for growth. When triticale is harvested as triticale whole crop silage the DM yield ranges between 9 and 11 ton of dry matter per hectare. Therefore, under water limiting conditions it may be attractive to replace forage maize by triticale whole crop silage. The objective of this study is to obtain information about the effects of replacing maize silage by triticale whole crop silage on feed intake and milk production by dairy cows Materials and methods Two similar feeding trials were conducted in the winter season of 1996/1997 and 1997/1998 respectively. -

Feeding Rye Or Triticale to Dairy Cattle
Feeding Rye or Triticale to Dairy Cattle Liz Binversie | Agriculture Educator | Extension Brown County Matt Akins | Assistant Scientist and Extension Specialist | Marshfield Agriculture Research Station Kevin Shelley |Extension NPM Specialist | UW-Madison Dept. of Horticulture Randy Shaver | Emeritis professor | UW-Madison Dept. of Dairy Science Introduction Use of cereal grain forages, such as rye and triticale gains. However, some farms may not raise (hybrid of rye and wheat), has become an increasingly youngstock on site and will need to consider if they important topic, and is especially relevant during should incorporate cereal forages in the dry or years when feed inventory is short. However, it is not lactating cow diets, and if so, how much to feed. The without challenges. Timing can conflict with higher farm should assess its current feed inventory to priority tasks on the farm such as alfalfa harvest and determine cereal forage needs, which may be helpful corn planting in spring, and corn silage harvest and in determining the acreage that should be planted manure application in the fall. Therefore establishing and harvested. Estimated yield is about 1 to 2 tons of and harvesting rye or triticale can be a challenge. dry matter per acre, if harvested at boot stage for Achieving optimal quality for lactating cows can be optimal quality to feed to lactating cows. Some farms another issue because quality declines can happen may only want to grow enough to feed for part of the quickly. Farmers, and their nutritionists, have shared year, while others may prefer to have enough ryelage that they need data and guidance to make good inventory to feed all year round. -

Glucosyltransferase B3GLCT Gene Mutated in Peters Plus Syndrome
RESEARCH ARTICLE Functional characterization of zebrafish orthologs of the human Beta 3- Glucosyltransferase B3GLCT gene mutated in Peters Plus Syndrome Eric Weh1,2¤a, Hideyuki Takeuchi3¤b, Sanaa Muheisen1, Robert S. Haltiwanger3, Elena V. Semina1,2,4* 1 Department of Pediatrics and Children's Research Institute at the Medical College of Wisconsin, a1111111111 Milwaukee, Wisconsin, United States of America, 2 Department of Cell Biology, Neurobiology, and Anatomy at the Medical College of Wisconsin, Milwaukee, Wisconsin, United States of America, 3 Complex a1111111111 Carbohydrate Research Center, University of Georgia, Athens, Georgia, United States of America, a1111111111 4 Department of Ophthalmology at the Medical College of Wisconsin, Milwaukee, Wisconsin, United States a1111111111 of America a1111111111 ¤a Current address: Department of Ophthalmology and Visual Sciences, W.K. Kellogg Eye Center, University of Michigan, Ann Arbor, MI, United States of America ¤b Current address: Department of Molecular Biochemistry, Nagoya University School of Medicine, Nagoya, Japan. * [email protected] OPEN ACCESS Citation: Weh E, Takeuchi H, Muheisen S, Haltiwanger RS, Semina EV (2017) Functional Abstract characterization of zebrafish orthologs of the human Beta 3-Glucosyltransferase B3GLCT gene Peters Plus Syndrome (PPS) is a rare autosomal recessive disease characterized by ocular mutated in Peters Plus Syndrome. PLoS ONE 12 defects, short stature, brachydactyly, characteristic facial features, developmental delay (9): e0184903. https://doi.org/10.1371/journal. pone.0184903 and other highly variable systemic defects. Classic PPS is caused by loss-of-function muta- tions in the B3GLCT gene encoding for a 3-glucosyltransferase that catalyzes the attach- Editor: Christoph Englert, Leibniz Institute on aging β - Fritz Lipmann Institute (FLI), GERMANY ment of glucose via a β1±3 glycosidic linkage to O-linked fucose on thrombospondin type 1 repeats (TSRs). -

Triticale Forage Productivity and Use
Excerpt from the Triticale Manual Print copies are available from [email protected] Triticale Production and Utilization Manual 2005 Spring and Winter Triticale for Grain, Forage and Value-added Alberta Agriculture, Food and Rural Development Part 4. Triticale for Forage Part 4. TRITICALE FOR FORAGE Triticale forage productivity and use Annual cereals can provide an excellent source of In these applications, forage triticale has much to supplementary forage, offering an extended offer in helping diversify Western Canadian grazing season and diversity in crop rotations cropping systems (Briggs, 2001; The Growth (Figure 4). Due to its superior silage yield Potential of Triticale in Western Canada). potential, triticale has proven to be very competitive with other cereals for yield and For detailed information on triticale forage quality. production, refer to the AgDex ‘Triticale Forage Manual’ (2005, in press). An advantage of growing winter triticale is its extension of early spring and late fall grazing. Figure 4. Seasonal distribution of pasture yields of annuals Fall seeded winter cereal 50 Barley 40 Spring seeded winter cereal 30 % of total annual Oats yield 20 10 0 Early Mid Late Mid Mid June June July August Sept (adapted from A. Aasen, Western Forage/Beef Group, Lacombe ) - 25 - Part 4. Triticale for Forage Triticale silage Winter triticale for silage Some quick facts about triticale silage production and quality: Fall-planted winter cereals such as triticale and rye provide a valuable source of forage when Spring triticale for silage spring grazed prior to being harvested for silage or seed. Rotating winter cereals with Spring triticale for silage is competitive with barley silage crops offers breaks for disease other silage options in Western Canada and in and pest control. -

Evaluation of Triticale Grain in High Producing Dairy Cow Rations
AN ABSTRACT OF THE THESIS OF Robert L. Biedenbach, Jr. for the degree of Master of Science in Animal Science presented on September 29, 1989. Title: Evaluation of Triticale Grain in High Producina Dairy Cow Rations Abstract approved by:Redacted for Privacy The objective of this study was to evaluate triticale grain fed as a concentrate in the diet of high producing dairy cows. A lactation study, in vitro dry matter digestibilities and a preliminary physiological effects trial were conducted to evaluate triticale and used to make feeding recommendations. In the lactation study, thirty-two multiparous Holstein cows were divided into two groups based on days in milk and initial milk production. A switchback design was employed. Diets were isocaloric, isonitrogenous, and isofibrous total mixed rations. Triticale replaced barley in the experimental ration. Daily feedings were recorded and orts were weighed back biweekly. No significant differences were noted in five day 4% fat corrected milk weights or daily average milk weight for triticale and control diets, 385.27 and 388.47 pounds, 77.05 and 77.69 pounds, respectively, when triticale replaced barley on an equal weight basis. Triticale supported a peak production for a five day period averaging 114.25 lbs. per day and the control ration a peak five day average production of 109.30 lbs. per day. No significant difference was noted in % milk protein as treatment means were 3.14 and 3.16% for triticale and the control ration. However, five day weights for protein production were significantly higher (P<.05) for animals fed the control TMR (13.09 lbs.) than the animals fed the triticale TMR (12.45 lbs.). -

2019 Abstracts
We are delighted EDITORIAL to welcome the worldwide yeast community in Gothenburg for ICYGMB2019! The “International Yeast Conferences” started in the 1960s with a handful of delegates and since then have become THE most important event in yeast research. Now the yeast meeting to returns to Gothenburg. Many yeast researchers still remember the meeting in 2003 with over 1,100 delegates, a truly memorable event. The Life Sciences are changing, and yeast research remains at their forefront. Advancements in genome sequencing and genome editing just make yeast more exciting as model organism in basic cell biological research, genome evolution and as a tool for synthetic biology and biotechnology. One of the most important reasons for the enormous success of yeast research lies in the unique character of the international yeast research community. No other community employs such a free exchange and access to information and research tools. Nor has any other community had the ability to build – even intercontinental – consortia of critical mass to tackle large‐scale projects, such as in sequencing the first eukaryotic genome or the first comprehensive yeast knockout library. Yeast2019 is the meeting of the international yeast research community where the latest, and even unpublished results are exchanged, and new projects, alliances, and collaborations are founded. A do‐not‐miss‐event. We attempt to incorporate the present excitement in yeast research in the programme of yeast2019. We are confident that this conference will contain important news and information for all yeast researchers. Taken together, yeast2019 will provide an up‐ to‐date overview in yeast research and it will set the scene for years to come. -
![Genetic Variation, Heritability and Path-Analysis in Ethiopian Finger Millet [Eleusine Coracana (L.) Gaertn] Landraces](https://docslib.b-cdn.net/cover/4681/genetic-variation-heritability-and-path-analysis-in-ethiopian-finger-millet-eleusine-coracana-l-gaertn-landraces-994681.webp)
Genetic Variation, Heritability and Path-Analysis in Ethiopian Finger Millet [Eleusine Coracana (L.) Gaertn] Landraces
Kasetsart J. (Nat. Sci.) 40 : 322 - 334 (2006) Genetic Variation, Heritability and Path-Analysis in Ethiopian Finger Millet [Eleusine coracana (L.) Gaertn] Landraces Kebere Bezaweletaw1, Prapa Sripichitt2*, Wasana Wongyai2 and Vipa Hongtrakul3 ABSTRACT A total of 66 finger millet accessions constituted of 64 landraces and two standard varieties were evaluated for 15 morpho-agronomic characters in randomized complete block design with three replications at Aresi-Negele Research Sub-Center in Ethiopia during 2004 main cropping season. The objectives were to assess the variability and association of characters. The mean squares of genotypes were highly significant for all characters. Relatively, grain yield per plant exhibiting the highest range (4.87-21.21g) and days to maturity showed the lowest range (143-200 days) of 336 and 40% where maximal values were greater than the corresponding minimal values, respectively. For all characters, the phenotypic and genotypic coefficients of variations varied in the orders of 8.05-31.23% and 6.52- 24.21% in both cases for days to maturity and grain yield per plant, respectively. Heritability estimates ranged from 20% for grain-filling duration to 84% for days to heading. Values of expected genetic advance varied from 6.67-44.14% for grain-filling duration and finger width, respectively. Finger width and length exhibited high heritability coupled with high genetic advance. The strongest positive association was observed between culm thickness and leaf blade width while the strongest negative association was found between 1,000-grain weight and finger number. Grain yield per plant associated positively with productive tillers, 1,000-grain weight, the number of grains per spikelet and finger number and negatively associated with days to heading and maturity.