Molecular Advances for Improving Nutrient Bioavailability
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Cadmium, Lead, Zinc and Copper Levels in Selected Ecological
DOI: 10.1515/cipms-2017-0027 Curr. Issues Pharm. Med. Sci., Vol. 30, No. 3, Pages 147-150 Current Issues in Pharmacy and Medical Sciences Formerly ANNALES UNIVERSITATIS MARIAE CURIE-SKLODOWSKA, SECTIO DDD, PHARMACIA journal homepage: http://www.curipms.umlub.pl/ Evaluation of cadmium, lead, zinc and copper levels in selected ecological cereal food products and their non-ecological counterparts Katarzyna Slepecka*, Klaudia Kalwa, Jakub Wyrostek, Urszula Pankiewicz Department of Analysis and Evaluation of Food Quality, Faculty of Food Science and Biotechnology, University of Life Sciences in Lublin, Skromna 8, 20-704 Lublin, Poland ARTICLE INFO ABSTRACT Received 07 September 2017 In the everyday human diet, cereal products are considered to be basics. Such food should Accepted 05 October 2017 have healthy properties and not contain harmful additives, especially heavy metals as Keywords: exposure to low doses of such xenobiotics can adversely affect human health. Ecological cereal products, farming is the answer to consumer expectations regarding food safety, and ecological heavy metals, products are recommended as a basis for proper nutrition, despite the higher cost of their ecological and non-ecological products. purchase. The present study was carried out to evaluate the content of heavy metals in ecological cereal products and their non-ecological analogues. INTRODUCTION According to the Polish Food Pyramid, the share of cereal (copper, zinc, cobalt, iron, manganese, selenium, molyb- products in the daily diet is significant and thus forms the denum) act harmlessly after exceeding certain amounts basis of healthy human nutrition [1]. What is more, such specified in the standards [3]. Contamination of food with cereal products should be of the minimum degree of pro- heavy metals comes from pollution of soil, water and air, cessing, e.g cereal grains, meal grains, grits, flour, bran and mainly from industry, transportation and agricultural chem- embryos. -

Final Seed Times
FODDER AND PSEUDOCEREAL SEED PRODUCTION (STATUS, CONSTRAINT, AND STRATEGIES) Volume 13 No. 2, May - Aug 2020 The National Seed Association of India Magazine FODDER AND PSEUDOCEREAL SEED PRODUCTION (Status, Constraint and Strategies) SEED TIMES 1 May - August 2020 FODDER AND PSEUDOCEREAL SEED PRODUCTION (STATUS, CONSTRAINT, AND STRATEGIES) 2 SEED TIMES May - August 2020 FODDER AND PSEUDOCEREAL SEED PRODUCTION (STATUS, CONSTRAINT, AND STRATEGIES) ABOUT NSAI National Seed Association of India (NSAI) is the apex a wide range of agro-climatic zones. It actively contributes to organization representing the Indian seed industry. The vision the seed industry policy development, with the concerned of NSAI is to create a dynamic, innovative and internationally governments, to ensure that policies and regulations create an competitive, research based industry producing high enabling environment, including public acceptance, so that the performance, high quality seeds and planting materials which industry is globally competitive. benefit farmers and significantly contribute to the sustainable growth of Indian Agriculture. NSAI promotes harmonization and adoption of best commercial practices in production, processing, quality control and The mission of NSAI is to encourage investment in state of distribution of seeds. the art R&D to bring to the Indian farmer superior genetics and technologies, which are high performing and adapted to NSAI Office Bearers NSAI Governing Council Members President: Mr. M. Prabhakar Rao Mr. N.P. Patel Mr. K. Praveen Kumar Nuziveedu Seeds Ltd. Western Agri Seeds Ltd Asian Agri Genetics Ltd. Vice President: Mr. Kamal O. Zunzunwala Mr. Arun Kumar Agarwalla Mr. Siddhartha S Sen Safal Seeds & Biotech Ltd West Bengal Hybrid Seeds Parasmoni Organic & Agri Products Mr. -

Downloaded on 12 March 2021, Was Applied to Evaluate the Extent of Species Other Than Chia in RNA-Seq Assemblies
plants Article Proteomic Identification and Meta-Analysis in Salvia hispanica RNA-Seq de novo Assemblies Ashwil Klein 1 , Lizex H. H. Husselmann 1 , Achmat Williams 1, Liam Bell 2 , Bret Cooper 3 , Brent Ragar 4 and David L. Tabb 1,5,6,* 1 Department of Biotechnology, University of the Western Cape, Bellville 7535, South Africa; [email protected] (A.K.); [email protected] (L.H.H.H.); [email protected] (A.W.) 2 Centre for Proteomic and Genomic Research, Cape Town 7925, South Africa; [email protected] 3 USDA Agricultural Research Service, Beltsville, MD 20705, USA; [email protected] 4 Departments of Internal Medicine and Pediatrics, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02150, USA; [email protected] 5 Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town 7500, South Africa 6 Centre for Bioinformatics and Computational Biology, Stellenbosch University, Stellenbosch 7602, South Africa * Correspondence: [email protected]; Tel.: +27-82-431-2839 Abstract: While proteomics has demonstrated its value for model organisms and for organisms with mature genome sequence annotations, proteomics has been of less value in nonmodel organisms that are unaccompanied by genome sequence annotations. This project sought to determine the value of RNA-Seq experiments as a basis for establishing a set of protein sequences to represent a nonmodel organism, in this case, the pseudocereal chia. Assembling four publicly available chia RNA-Seq datasets produced transcript sequence sets with a high BUSCO completeness, though the Citation: Klein, A.; Husselmann, number of transcript sequences and Trinity “genes” varied considerably among them. -

The Probable Use of Genus Amaranthus As Feed Material for Monogastric Animals
animals Review The Probable Use of Genus amaranthus as Feed Material for Monogastric Animals Tlou Grace Manyelo 1,2 , Nthabiseng Amenda Sebola 1 , Elsabe Janse van Rensburg 1 and Monnye Mabelebele 1,* 1 Department of Agriculture and Animal Health, College of Agriculture and Environmental Sciences, University of South Africa, Florida 1710, South Africa; [email protected] (T.G.M.); [email protected] (N.A.S.); [email protected] (E.J.v.R.) 2 Department of Agricultural Economics and Animal Production, University of Limpopo, Sovenga 0727, South Africa * Correspondence: [email protected]; Tel.: +27-11-471-3983 Received: 13 July 2020; Accepted: 5 August 2020; Published: 26 August 2020 Simple Summary: In monogastric production, feeds account for about 50–70% of the total costs. Protein ingredients are one of the most expensive inputs even though they are not included in large quantities as compared to cereals. Monogastric animal industries are faced with a major problem of limited protein sources, moreover, the competition for plant materials is expected to further increase feed prices. Therefore, to tackle this problem, interventions are required to find alternative and cost-effective protein sources. One identified crop that meets these requirements is amaranth. Studies have shown the potential and contribution that amaranth has as an alternative ingredient in diets for monogastric animals. Therefore, the main purpose of this review is to provide a detailed understanding of the potential use of amaranth as feed for monogastric animals, and further indicate processing techniques are suitable to improve the utilization of grain amaranth and leaves. -

Chenopodium Pallidicaule Aellen)
Crimson Publishers Mini Review Wings to the Research Underutilized Andean Crop Kañawa (Chenopodium Pallidicaule Aellen) Juan Pablo Rodriguez1* and Marten Sørensen2 1International Center for Biosaline Agriculture, UAE 2Department of Plant & Environmental Sciences, University of Copenhagen, Denmark ISSN: 2637-7802 Abstract Small farmers worldwide are the custodians of agro-biodiversity belonging to both the plant and animal kingdoms. Grains and vegetables are the essentials needed to sustain our food systems. Goosefoots, i.e., Chenopodium species like kañawa (Ch. pallidicaule) and Quinoa (Ch. quinoa), are prominent examples of domestication by small farmers during ancient times that still exist. Chenopodium grains possess high ñawa tolerates salinity, drought and frost and its diversity allows farmers to cultivate the crop even above 4000m a.s.l. It isnutritional a staple food profiles source and as arean ingredient further characterized in a balanced by and being low glycaemicresilient climateindex diet. crops. Ka Introduction Kañawa, also known as cañahua or cañihua (Ch. pallidicaule Aellen), is a close relative of quinoa (Ch. quinoa Willd.) originating in the Peruvian and Bolivian Andean Highlands or Puna. Bolivia and Peru conserve a large collection of cañahua accessions with 801 and 341 entries, respectively [1-3]. While the quinoa is cultivated widespread both commercially *Corresponding author: Juan Pablo Rodriguez, Department of Plant & and experimentally, the kañawa is cultivated on a small-scale by farmers in both countries Environmental Sciences, University of [4]. The grain is an achene fruit like that of the quinoa and small-sized and with a very low Copenhagen, Frederiksberg C, Denmark saponin content and a high nutritional value that surpasses that of the quinoa. -

Seed Dormancy and Preharvest Sprouting in Quinoa (Chenopodium Quinoa Willd)
plants Review Seed Dormancy and Preharvest Sprouting in Quinoa (Chenopodium quinoa Willd) Emma M. McGinty 1, Kevin M. Murphy 2 and Amber L. Hauvermale 2,* 1 The School of Biological Sciences, Washington State University, P.O. Box 644236, Pullman, WA 99164, USA; [email protected] 2 Department of Crop and Soil Science, Washington State University, Pullman, WA 99164, USA; [email protected] * Correspondence: [email protected]; Tel.:+1-509-335-3661 Abstract: Quinoa (Chenopodium quinoa Willd.) is a culturally significant staple food source that has been grown for thousands of years in South America. Due to its natural drought and salinity tolerance, quinoa has emerged as an agronomically important crop for production in marginal soils, in highly variable climates, and as part of diverse crop rotations. Primary areas of quinoa research have focused on improving resistance to abiotic stresses and disease, improving yields, and increasing nutrition. However, an evolving issue impacting quinoa seed end-use quality is preharvest sprouting (PHS), which is when seeds with little to no dormancy experience a rain event prior to harvest and sprout on the panicle. Far less is understood about the mechanisms that regulate quinoa seed dormancy and seed viability. This review will cover topics including seed dormancy, orthodox and unorthodox dormancy programs, desiccation sensitivity, environmental and hormonal mechanisms that regulate seed dormancy, and breeding and non-breeding strategies for enhancing resistance to PHS in quinoa. Citation: McGinty, E.M.; Murphy, Keywords: abscisic acid; desiccation sensitivity; gibberellin; hormone signaling; precocious germina- K.M.; Hauvermale, A.L. Seed tion; seed morphology Dormancy and Preharvest Sprouting in Quinoa (Chenopodium quinoa Willd). -

SRP472 3 Comparison of Rye and Triticale As Forages for Grazing
Selected Articles from 1985 Report of Agricultural Research Southeast Branch Experiment Station Agricultural Experiment Station Kansas State University 16 Comparison of Rye and Triticale as Forages for Grazing Stocker Cattle Winter annual small grains are frequently grazed during late fall and early spring in southeastern Kansas. Wheat is often the crop of choice, especially if grain production is the primary objective. If pasture is the main consideration, there are probably other small grains that will yield more forage and produce a greater quantity of beef cattle weight gain per acre than wheat. Research has been conducted at the Southeast Kansas Experiment Station to determine which winter annual small grains will result in maximum forage and beef production in a graze-out program. A study conducted in 1981-82 revealed that triticale produced nearly twice as much beef liveweight gain per acre as Newton wheat. Results from a 1982-83 study indicated that a mixture of 2/3 rye and 1/3 wheat produced over three times as much beef liveweight per acre as triticale. The following study was conducted to compare rye and triticale with respect to performance of grazing stocker cattle. Procedure: On September 19, 1983, two 5-acre fields were seeded with winter annuals. One field was seeded with 105 lb of triticale per acre and the other was seeded with 89 lb of Bonel rye per acre. At seeding time, 25-65-70 lb of N-P2O5-K2O per acre was applied and on November 14, 1984, 50 lb of N per acre was applied to each pasture. -

An Overview of Buckwheat (Fagopyrum Spp)-An Underutilized
Available online at www.ijmrhs.com al R edic ese M a of rc l h a & n r H u e o a J l l t h International Journal of Medical Research & a n S ISSN No: 2319-5886 o c i t i Health Sciences, 2020, 9(7): 39-44 e a n n c r e e t s n I • • I J M R H S An Overview of Buckwheat (Fagopyrum spp)-An Underutilized Crop in India-Nutritional Value and Health Benefits Aneesha Nalinkumar* and Pratibha Singh Department of Nutrition and Dietetics, Manav Rachna International Institute of Research and Studies, Haryana, India *Corresponding e-mail: [email protected] ABSTRACT Buckwheat is one of the pseudocereals grown annually in hilly regions of India. It belongs to the family Polygonaceae and genus (Fagopyrum spp.) Buckwheat is adaptable to extreme cold temperatures, stress conditions of water, less soil fertility and varying climatic conditions, making it a sustainable crop. A literature search on Buckwheat was done using PubMed and Google search engines and reviewed to prepare an overview of its cultivation, nutritional and health value. In India, twenty species of Buckwheat are cultivated across various hilly regions. Out of these only nine species have desirable nutritional value and two are commonly grown. They are Fagopyrum esculentum Moench (Common buckwheat) and Fagopyrum tataricum (Tartary buckwheat). However, the cultivation of Buckwheat has declined in the 20th century making it an underutilized crop. Buckwheat has good amount of nutrients and many health benefits. There is a need to research about this under-utilized crop and create awareness as this crop has many nutritional and health benefits. -

Development of New Starch Formulations for Inclusion in the Dietotherapeutic Treatment of Glycogen Storage Disease †
Proceedings Development of New Starch Formulations for Inclusion in the Dietotherapeutic Treatment of Glycogen Storage Disease † Raquel Selma-Gracia 1,2, José Moisés Laparra Llopis 2 and Claudia Monika Haros 1,* 1 Instituto de Agroquímica y Tecnología de Alimentos (IATA), Consejo Superior de Investigaciones Científicas (CSIC), Av. Agustín Escardino 7, Parque Científico, 46980 Paterna, Valencia, Spain; [email protected] 2 Molecular Immunonutrition Group, Madrid Institute for Advanced Studies in Food (IMDEA-Food), Ctra. de Canto Blanco n° 8, 28049 Madrid, Spain; [email protected] * Correspondence: [email protected] † Presented at the 2nd International Conference of Ia ValSe-Food Network, Lisbon, Portugal, 21–22 October 2019. Published: 4 August 2020 Abstract: In this study, the thermal properties of quinoa and maize starch were evaluated and related to their digestibility. Lower gelatinisation and retrogradation parameters were obtained in quinoa starch, suggesting a better susceptibility to the disruption of the crystalline structure. These results were accompanied with a higher percentage of hydrolysis in raw quinoa, reaching more twofold higher than in raw maize starch. Besides, the slopes calculated by a Lineweaver-Bürke transformation showed similar values in raw quinoa and maize starches. Taken together, these characteristics of quinoa starch could provide more digestible benefits than the current treatment, raw maize starch, in glycogen storage disease patients. Keywords: maize starch; quinoa starch; thermal properties; starch hydrolysis; glycogen storage disease 1. Introduction Previous research has shown variability in the susceptibility to digestion depending on structural differences in starches from different sources [1]. A crystalline structure is an important factor to take into account in digestibility, and can be modified by a gelatinisation process [2]. -

Regular Article Effect of Addition of Spelt And
REGULAR ARTICLE EFFECT OF ADDITION OF SPELT AND BUCKWHEAT HULL ON SELECTED PROPERTIES OF YOGHURT Agata Znamirowska1, Katarzyna Sajnar1, Magdalena Kowalczyk1, Maciej Kluz2, Magdalena Buniowska1* Address: 1University of Rzeszow, Faculty of Biology and Agriculture, Department of Dairy Technology, Ćwiklińskiej 2D, 35-601 Rzeszów, Poland, phone number: +48177854905. 2University of Rzeszow, Faculty of Biology and Agriculture, Department of Bioenergetics and Food Analysis, Ćwiklińskiej 1, 35-601 Rzeszów, Poland. *Corresponding author: [email protected] ABSTRACT Buckwheat and spelled hulls are little tested in their use in the production of functional foods. The aim of this study was to evaluate the use of hull as a functional additive in yoghurt and to determine the effect of various doses of buckwheat and spelt hull on the physicochemical, organoleptic and microbiological properties of yoghurt. The enrichment of yoghurt with buckwheat and spelled hull resulted in a decrease in total acidity and a decrease in syneresis. Addition of spelt and buckwheat hulls to yoghurts reduced the colour brightness and increased the intensity of the red and yellow colours. The highest number of S. thermophilus was found in yoghurt containing 3% buckwheat hull and the beneficial effect of the addition of hull on L. bulgaricus growth was demonstrated. The type of hull determined the flavour and odour of yoghurts. Spelt hull gave the yoghurts a more intense floury and grainy flavour than buckwheat hull. Keywords: yoghurt, buckwheat hull, spelt hull, dietary fibre INTRODUCTION Food industry companies have high expectations in terms of food products that meet the consumers’ demand for a healthy lifestyle. In this context, functional food plays a special role, as apart from its fundamental goal, which is nutrition, it also has the psychological or physiological impact on the human body (Menrad, 1990). -

Article on Genetic Markers for Bunt Resistance From
Let’s make grain great again Click here to sign up for the newsletter The Landrace Newsletter no. 5 May 2021 A new growing season is ahead of us, and I greet the spring with news from both future and past from the organic grain sector. I wish you joyfull reading Anders Borgen Content in this newsletter Open field day and general assembly in Landsorten, Tuesday 22. June..............................................2 But now then, is it Landsorten or Agrologica, selling organic seed in future?...................................2 Mobile stone mill for local production................................................................................................3 Nordic grain festival 28th-30th October 2021 in Norway.....................................................................4 News from Agrologica science lab......................................................................................................4 Genetic markers for bunt resistance - news from LIVESEED-, Økosort-II and bunt projects......4 Acid rain and gluten-index..............................................................................................................4 Zanduri, Macha, and the hailstorm in Georgia...............................................................................6 Colchic emmer (Triticum paleochochicum)...............................................................................6 Emmer........................................................................................................................................7 Durum........................................................................................................................................7 -
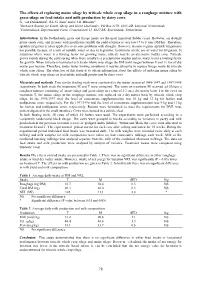
78 the Effects of Replacing Maize Silage by Triticale Whole Crop Silage
The effects of replacing maize silage by triticale whole crop silage in a roughage mixture with grass silage on feed intake and milk production by dairy cows G. van Duinkerken1, R.L.G. Zom1 and E.J.B. Bleumer2 1Research Station for Cattle, Sheep and Horse Husbandry, PO Box 2176, 8203 AD, Lelystad, Netherlands 2Cranendonck, Experimental Farm, Cranendonck 11, 6027 RK, Soerendonk, Netherlands Introduction In the Netherlands, grass and forage maize are the most important fodder crops. However, on drought prone sandy soils, and in years with insufficient rainfall the yield of maize is very low (7 to 8 tons DM/ha). Therefore, sprinkle irrigation is often applied to overcome problems with drought. However, in some regions sprinkle irrigation is not possible because of a lack of suitable water or due to legislative restrictions on the use of water for irrigation. In situations where water is a limiting factor for growing maize, triticale may be an alternative fodder crop. Triticale grows mainly during the early spring when there usually is a precipitation surplus and so, water is not a limiting factor for growth. When triticale is harvested as triticale whole crop silage the DM yield ranges between 9 and 11 ton of dry matter per hectare. Therefore, under water limiting conditions it may be attractive to replace forage maize by triticale whole crop silage. The objective of this study is to obtain information about the effects of replacing maize silage by triticale whole crop silage on feed intake and milk production by dairy cows Materials and methods Two similar feeding trials were conducted in the winter season of 1996/1997 and 1997/1998 respectively.