Apis Mellifera L.) in Yield Response of Canola Under Rainfed Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
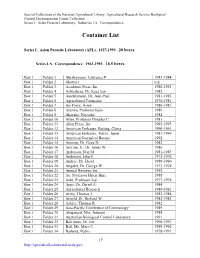
Container List
Special Collections of the National Agricultural Library: Agricultural Research Service Biological Control Documentation Center Collection Series I. Asian Parasite Laboratory. Subseries I.A. Correspondence. Container List Series I. Asian Parasite Laboratory (APL). 1927-1993. 20 boxes. Series I.A. Correspondence. 1963-1993. 16.5 boxes. Box 1 Folder 1 Abrahamson, Lawrence P. 1983-1984 Box 1 Folder 2 Abstract n.d. Box 1 Folder 3 Academic Press, Inc. 1986-1993 Box 1 Folder 4 Achterberg, Dr. Kees van 1982 Box 1 Folder 5 Aeschlimann, Dr. Jean-Paul 1981-1985 Box 1 Folder 6 Agricultural Counselor 1976-1981 Box 1 Folder 7 Air Force, Army 1980-1981 Box 1 Folder 8 Aizawa, Professor Keio 1986 Box 1 Folder 9 Akasaka, Naoyuki 1982 Box 1 Folder 10 Allen, Professor Douglas C. 1981 Box 1 Folder 11 Allen Press, Inc. 1992-1993 Box 1 Folder 12 American Embassy, Beijing, China 1990-1991 Box 1 Folder 13 American Embassy, Tokyo, Japan 1981-1990 Box 1 Folder 14 American Journal of Botany 1992 Box 1 Folder 15 Amman, Dr. Gene D. 1982 Box 1 Folder 16 Amrine, Jr., Dr. James W. 1986 Box 1 Folder 17 Anderson, Don M. 1981-1983 Box 1 Folder 18 Anderson, John F. 1975-1976 Box 1 Folder 19 Andres, Dr. Lloyd 1989-1990 Box 1 Folder 20 Angalet, Dr. George W. 1973-1978 Box 1 Folder 21 Annual Reviews Inc. 1992 Box 1 Folder 22 Ao, Professor Hsien-Bine 1988 Box 1 Folder 23 Aoki, Professor Joji 1977-1978 Box 1 Folder 24 Apps, Dr. Darrel A. 1984 Box 1 Folder 25 Agricultural Research 1980-1981 Box 1 Folder 26 Army, Thomas J. -

View the PDF File of the Tachinid Times, Issue 18
The Tachinid Times ISSUE 18 February 2005 Jim O’Hara, editor Invertebrate Biodiversity Agriculture & Agri-Food Canada C.E.F., Ottawa, Ontario, Canada, K1A 0C6 Correspondence: [email protected] The Tachinid Times continues to be offered in submissions, and the newsletter as a whole is reviewed hardcopy and online, with both versions having identical internally within my organization before it is posted on the pagination and appearance except that the figures in the Internet and distributed in hardcopy. Articles in The online version are produced in colour and the figures in the Tachinid Times are cited in Zoological Record. printed version are in black and white. The online version is available as a PDF file (ca. 1.5 MB in size) at the North Benno Herting, 1923–2004 (by H.-P. Tschorsnig) American Dipterists Society (NADS) website at: http:// At the age of 80 years, one of the most outstanding www.nadsdiptera.org/Tach/TTimes/TThome.htm. experts on tachinids, Dr. Benno Herting, died in Freiberg I would like to dedicate this issue of The Tachinid am Neckar (south-western Germany) on 19 July 2004. Times to a leader in tachinid systematics who passed away Benno Wilhelm Herting was born on 30 December in 2004, Dr. Benno Herting. His high standards of research 1923 in Bochum (north-western Germany) as a single earned him a respected position among modern tachinid child of a Catholic schoolteacher. After he finished school, systematists. Through his papers, advice and identifications, he was forced to join the military. To his great fortune – he aided many researchers and students in systematics, according to his own words – he was released from the biological control, faunistics and ecology. -

Tachinid Flies (Diptera, Tachinidae) of Warsaw and Mazovia
POLISH ACADEMY OF SCIENCES • INSTITUTE OF ZOOLOGY MEMORABILIA ZOOLOGICA MEMORABILIA ZOOL. 35 141— 162 14SI AGNIESZKA DRABER-MOŃKO TACHINID FLIES (.DIPTERA, TACHINIDAE) OF WARSAW AND MAZO VIA ABSTRACT The Tachinidae of the Mazovian Lowland (285 species) account for 65% of the total number of species occurring in Poland. In Warsaw 164 species occur, including 123 in the suburbs, and 107 in urban green areas, the latter being subdivided into parks (90 species), green areas of housing estates (30 species) and the centre of the town (47 species). Such groups are represented by the highest number of species as parasitoids of lepidopterans and beetles, with high ecological amplitudes, and also polyphages with large geographical ranges (Palaearctic and Euro-Siberian). INTRODUCTION Flies of,the family Tachinidae are poorly known both in Poland and in the whole Palaearctic. Tachinid flies of Mazovia and Warsaw have not been extensively studied so far. From Warsaw 42 species were known [2, 8, 11]. At present 164 species have been recorded in Warsaw. From the Mazovian Lowland, 85 species have been recorded so far, including 23 species listed by Sznabl [11], nine species listed by Wiąckow- ski [13. 15]. three species quoted by Miczulski and Koślińska [7], and single species quoted by Kuntze [5], Pawłowicz [10], Koehler [4]. Szujecki [12], and the other species by Draber-Mońko [2,8]. At present 285 ta,chinid flies are known from the Mazovian Lowland. The objective of the work was to establish the species composition of tachinid flies living in the Mazovian Lowland and Warsaw, and to analyse their geographical distribution and ecology. -

Tachinidae Bigot 1853
Dr Francesco Fiume Tachinidae Bigot 1853 Taxonomy of Tachinidae family Clade Natura Clade Mundus Plinius Superdominium/Superdomain Biota Bernard Pelletier 2012 Domain Eukaryota (Chatton 1925) Whittaker et Margulis 1978 Clade Amorphea Adl 2005 Clade Opisthokonta (Cavalier Smith 1987) Adl 2005 Clade Holozoa Lang et al . 2002 Kingdom Animalia Linnaeus 1758 Clade Epitheliozoa Ax 1996 Subkingdom Eumetazoa Bütschli 1910 Clade Bilateria Hatschek 1888 Clade Eubilateria Ax 1987 or (synonym) Nephrozoa Jondelius et al. 2002 Clade Protostomia Grobben 1908 Clade Ecdysozoa Aguinaldo et al . 1997 Superphylum Panarthropoda Nielsen 1995 Phylum Arthropoda von Siebold 1848 Clade Euarthropoda Lankester 1904 Clade Mandibulata Snodgrass 1938 Clade Crustaceomorpha Chernyshev 1960 Clade Labrophora Siveter, Waloszek et Williams 2003 Subphylum Pancrustacea Zrzavý et al . 1997 Clade Altocrustacea Regier et al . 2010 Clade Miracrustacea Regier et al . 2010 Superclass Exapoda Latreille 1825 Class Insecta Linnaeus 1758 Clade Dicondylia Hennig 1953 Subclass Pterygota Lang 1888 Infraclass Neoptera van der Wulp 1890 Clade Eumetabola Hennig 1953 Clade Holometabola Heider 1889 or (synonym) Endopterygota Sharp 1898 Superordo Panorpida Kristensen 1981 or (synonym) Mecoptera Hyatt et Arms 1891 Clade Antliophora Henning 1969 Order Diptera Linnaeus 1758 Suborder Brachycera Schiner 1862 Section Cyclorrhapha Brauer 1863 Infraorder Muscomorpha McAlpine 1989 Section Schizophora Becher 1882 Subsection Calyptratae Robineau-Desvoidy 1830 Superfamily Oestroidea Latreille 1817 Family Tachinidae Bigot 1853. Generality The Tachinidae are a large and variable family of true flies within the insect order Diptera , with more than 8,200 known species and many more to be discovered. Over 1300 species have been described in North America alone. Insects in this family commonly are called tachina flies or simply tachinids. -
Tachinidae (Insecta, Diptera) of Saitama Prefecture
九州総合博物館研究報告第14号 Bulletin of the Kyushu University Museum No.14, 2016, pp.1-36 Tachinidae (Insecta, Diptera) of Saitama Prefecture 1) 2) Hiroshi SHIMA & Katsushi HARA 1)Kyushu University Museum, Kyushu University, Hakozaki, Fukuoka 812-8581, Japan. E-mail: [email protected] 2)327-1 Sakurazawa, Yorii-machi, Osato-gun, Saitama 369-1201, Japan. Abstract: A total of 341 species of the Tachinidae (Diptera) are recorded from Saitama Prefecture, central Japan (Honshu). Seventeen species are newly recorded from Japan (Billaea brevicauda Zhang & Shima, B. kolomietzi (Mesnil), Istocheta rufipes (Villeneuve), Carcelia townsendi Crosskey, C. delicatula Mesnil, Catagonia aberrans (Mesnil), Senometopia longie- pandriuma (Chao & Liang), S. mimoexcisa (Chao & Liang), Blepharipa tibialis (Chao), Onychogonia flaviceps (Zetterstedt), Pales peregrina Herting, Cylindromyia arator Reinhard, Hemyda hertingi Ziegler & Shima, Perigymnosoma globulum Villeneuve, Panzeria vagans (Meigen), Glaurocara lucidula Richter, and Peleteria sphyricera (Macquaert)) and five genera each with an undetermined species are newly known from Japan (Admontia, Ligeria, Medinodexia, Hapalioloemus, and Phytomyptera). Exorista (Adenia) tubigera Mesnil and Exorista (Spixomyia) lepis Chao, which were mistakenly omitted in the Catalog of the Insects of Japan (Shima, 2014), are noted. Key Word: Tachinid fauna, Saitama Prefecture, Japanese Tachinidae, parasitoids, systematics. 1. Introduction Prefecture in Honshu based on material donated to the Kyushu University Museum together with material reported by Hara The Tachinidae are one of the largest families of the (1978). Seventeen species are newly recorded from Japan. Five Diptera and are known from some 8500 species in the world genera are also newly recorded from Japan, although the (O’Hara, 2013: 1). All members of the family develop as endo- species are not yet determined. -
Historical Records of Tachinidae (Diptera) from Lithuania
NAUJOS IR RETOS LIETUVOS VABZDŽI Ų R ŪŠYS. 22 tomas 133 HISTORICAL RECORDS OF TACHINIDAE (DIPTERA) FROM LITHUANIA ERIKAS LUTOVINOVAS Department of Botany and Zoology, Masaryk University, Kotlá řská 2, CZ-611 37 Brno, the Czech Republic. E-mail: [email protected] Introduction The population size of insect parasitoids may vary in a great amplitude; therefore, historical collections may provide species of these insects fairly possibly be collected from some areas during decades. The global warming enables species spreading to the north, though very frequently we are unable to say whether the newly found species have been living in the area before because of a short tradition in Lithuanian Dipterology where Tachinidae are poorly represented in the collections. Valuable specimens were found in the collections of historical entomologists, that are preserved at Vilnius University and at T. Ivanauskas Zoological Museum in Kaunas. Some part of those findings have been published before (Lutovinovas, 2006), the rest are provided in the present study. Material and methods The studied material was collected in 1926–1929. Specimens collected in the territory of the then Lithuania are deposited in the collection of T. Ivanauskas Zoological Museum (Kaunas); consequently, those collected in the Vilnius Region (which was occupied by Poland at that time), are deposited at the Museum of Zoology of Vilnius University (Vilnius). Marja Ostrejkówna (M.O.), who has collected the material in the Vilnius Region, swept insects from the vegetation (exact information is provided in the notebook preserved at Vilnius University), while no information is available about the methods implemented by Alfonsas Palionis (A.P.), who collected insects from the territory of the then Lithuania. -

Landscape Dynamics of Tachinid Parasitoids
UNIVERSITY OF PADOVA Department of Agronomy, Food, Natural Resources, Animals and Environment (DAFNAE) DOCTORAL SCHOOL OF CROP SCIENCES CURRICULUM CROP PROTECTION CYCLE XXVII Landscape dynamics of tachinid parasitoids Director of the School: Prof. Antonio Berti Supervisor: Dr. Lorenzo Marini Co-supervisors: Prof. Andrea Battisti Dr. Pierfilippo Cerretti PhD Student: Diego J. Inclán Luna Date of thesis submission January 30th 2015 Declaration I hereby declare that this submission is my own work and that, to the best of my knowledge and belief, it contains no material previously published or written by another person nor material which to a substantial extent has been accepted for the award of any other degree or diploma of the university or other institute of higher learning, except where due acknowledgment has been made in the text. Padova, 30 January 2015 Diego J. Inclán A copy of the thesis will be available at http://paduaresearch.cab.unipd.it/ Dichiarazione Con la presente affermo che questa tesi è frutto del mio lavoro e che, per quanto io ne sia a conoscenza, non contiene materiale precedentemente pubblicato o scritto da un'altra persona né materiale che è stato utilizzato per l’ottenimento di qualunque altro titolo o diploma dell'università o altro istituto di apprendimento, a eccezione del caso in cui ciò venga riconosciuto nel testo. Padova, 30 gennaio 2015 Diego J. Inclán Una copia della tesi sarà disponibile presso http://paduaresearch.cab.unipd.it/ Table of contents Summary………………………………………………………………..………… 7 Riassunto………………………………………………………………....………… -

View the PDF File of the Tachinid Times, Issue 26
THE TACHINID TIMES ISSUE 26 TTTTTT turns turns 25 25 with with a new a new look! look! Barcoding Finnish Tachinidae In quest of the Tachinid Phylogeny The pestiferous uzifly of India To 'Die Hel' and back Collecting in the Western Cape Where are all the genera? February 2013 Table of Contents Articles Progress towards a phylogeny of world Tachinidae. Year 1 4 by J.O. Stireman III, J.E. O’Hara, J.K. Moulton, P. Cerretti and I.S. Winkler Where in the world are all the tachinid genera? 10 by J.E. O’Hara DNA barcodes for north European Tachinidae: preliminary results and material request 17 by J. Pohjoismäki, J. Kahanpää and M. Mutanen To ‘Die Hel’ and back. Expeditions of the Phylogeny of World Tachinidae Project 20 Part I: Western Cape, South Africa by P. Cerretti, J.E. O’Hara, J.O. Stireman III, I.S. Winkler and A.H. Kirk-Spriggs A visit to the Vienna Museum with a brief history of the tachinid collection 30 by J.E. O’Hara Observations of Tachinidae (Diptera) in the surroundings 39 of Friedberg (Hessen, Germany) with notes on some interesting species by J. Pohjoismäki Metapopulation biology of the Indian uzifly, Exorista 46 sorbillans (Wiedemann) (Diptera: Tachinidae) by B.M. Prakash, A. Prathima, H. Ravikumar, H.C. Huchesh and H.P. Puttaraju Student News 50 Jeremy D. Blaschke 51 Zachary L. Burington 52 Book Announcement 53 Tachinid Bibliography 58 Mailing List Issue 26, 2013 The Tachinid Times February 2013, Issue 26 Message from the editor Chief Editor JAMES E. -

Miscellanea : Insects in Southern Rhodesian Tobacco Culture
Miscellanea : Insects in Southern Rhodesian tobacco culture. Part II, III, IV, Insects occurring in the fields : Hymenoptera ; Diptera ; Tenebrionidae and Elateridae Autor(en): Bünzli, G.H. / Büttiker, W.W. Objekttyp: Article Zeitschrift: Acta Tropica Band (Jahr): 14 (1957) Heft 3 PDF erstellt am: 02.10.2021 Persistenter Link: http://doi.org/10.5169/seals-310685 Nutzungsbedingungen Die ETH-Bibliothek ist Anbieterin der digitalisierten Zeitschriften. Sie besitzt keine Urheberrechte an den Inhalten der Zeitschriften. Die Rechte liegen in der Regel bei den Herausgebern. Die auf der Plattform e-periodica veröffentlichten Dokumente stehen für nicht-kommerzielle Zwecke in Lehre und Forschung sowie für die private Nutzung frei zur Verfügung. Einzelne Dateien oder Ausdrucke aus diesem Angebot können zusammen mit diesen Nutzungsbedingungen und den korrekten Herkunftsbezeichnungen weitergegeben werden. Das Veröffentlichen von Bildern in Print- und Online-Publikationen ist nur mit vorheriger Genehmigung der Rechteinhaber erlaubt. Die systematische Speicherung von Teilen des elektronischen Angebots auf anderen Servern bedarf ebenfalls des schriftlichen Einverständnisses der Rechteinhaber. Haftungsausschluss Alle Angaben erfolgen ohne Gewähr für Vollständigkeit oder Richtigkeit. Es wird keine Haftung übernommen für Schäden durch die Verwendung von Informationen aus diesem Online-Angebot oder durch das Fehlen von Informationen. Dies gilt auch für Inhalte Dritter, die über dieses Angebot zugänglich sind. Ein Dienst der ETH-Bibliothek ETH Zürich, Rämistrasse 101, 8092 Zürich, Schweiz, www.library.ethz.ch http://www.e-periodica.ch Insects in Southern Rhodesian Tobacco Culture.1 By G. IL Bünzli2 and W. W. Rüttiker2. Part II: Insects occurriiitc in the fields. HYMENOPTERA Inspections made of fields of Virginia Tobacco during the seasons 1949-1952 offered an opportunity of recording most of the insects which visit or dwell inside such tobacco fields. -

Title Flowering Phenology and Anthophilous Insect Community in a Grassland Ecosystem at Mt. Yufu, Western Japan Author(S)
Flowering Phenology and Anthophilous Insect Community in a Title Grassland Ecosystem at Mt. Yufu, Western Japan Author(s) YAMAZAKI, Kyoko; KATO, Makoto Contributions from the Biological Laboratory, Kyoto Citation University (2003), 29(3): 255 Issue Date 2003-04-21 URL http://hdl.handle.net/2433/156407 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Contr. biot. Lab. Kyoto Univ., Vol. 29, pp. 255-3 18, pl. 4-5 Issued 21 April 2003 Flowering Phenology and Anthophilous Insect Community in a Grassland Ecosystem at Mt. Yufu, Western Japan Kyoko YAMAZAKI and Makoto KATO Graduate School of Human and Environmenta1 Studies, Kyoto University, Yoshida-Nihonmatsu-cho, Sakyo-ku, Kyoto, 606-8501 Japan ABSTRACT The hillsides of Mt, Yufu, located in Kyusyu, Japan, is a dormant yolcano, are coyered with natural and semi-natural grasslands; the latter of which are maintained by traditional mowing and burning. Both the natura1 and semi-natura1 grasslands are inhabited by many grassland-specific plant species, some of which are now endangered in Japan. To understand pollination mutualisms in the grassland ecosystem, we investigated the flowering phenology and anthophilous insect communities on 149 plant species from 49 different plant families, from April to October 2001. In tota1, 1192 individuals from 308 species, 83 families and 10 orders of Insecta were observed on flowers of 101 plant species. The most abundant insect order was Hymenoptera (37.89o of individuals), followed by Diptera (32.59o), Coleoptera (22.79o) and Lepidoptera (6.29o), The proportions of Coleoptera and Lepidoptera were respectively smaller and greater than in forested habitats, suggesting that many anthophilous beetles depend on woody plants during their larval stages and that anthophilous butterflies (especially Nymphalidae) are associated with grassland-specific perennials (especially Viola spp.) in their larval stages. -

1 Introduction
Epsom Golf Course Management Plan: Incorporating Botanical & Invertebrate Surveys Written by: Scotty Dodd & Isobel Girvan BSc (Hons) MIEEM December 2010 Surrey Wildlife Trust, School Lane, Pirbright, Woking, Surrey GU24 0JN Contents 1.0 Introduction _________________________________________________________ 4 2.0 Site Description______________________________________________________ 5 3.0 Phase 1 Survey ______________________________________________________ 6 4.0 Conclusions ________________________________________________________ 16 5.0 Invertebrate Survey__________________________________________________ 19 8.0 Management Recommendations_______________________________________ 37 9.0 Botanical Species List _______________________________________________ 44 8.0 Invertebrate Species List _____________________________________________ 49 9.0 References _________________________________________________________ 57 10.0 Photographs _______________________________________________________ 60 Figure 1 Botanical Target Notes ___________________________________________ 62 Figure 2 Management Suggestions_________________________________________ 63 Appendix 1 ______________________________________________________________ 64 Photograph Title Page: Round-headed Rampion The contents of this report were correct at the time of the site visit. The report is provided for the sole use of the named client and is confidential. All rights in this report are reserved. No part of it may be reproduced or transmitted, in any form or by any means, electronic, mechanical, -

The in the UNITED STATES
5,i^frCfv^cí^S (^ <'^<^iUav 3 3, i-e. ^ Ax^-, ^^l The ■¿OVL in the UNITED STATES AGRICULTURE HANDBOOK NO. 236 Agricultural Research Service UNITED STATES DEPARTMENT OF AGRICULTURE CONTENTS Page How to recognize the Japanese beetle J The adult J The egg ^ The grub J The pupa . ^ Present distribution of the beetle in North Amenca 5 Probable ultimate distribution of the beetle in North America 6 Temperature and moisture requirements of the beetle 6 Western spread of the beetle 7 Southern spread of the beetle 8 Northern spread of the beetle 8 How the beetle spreads 8 Seasonal history and habits » Feeding by adult beetle 12 Type of feeding 12 Factors influencing feeding 12 Toxic plants 1* Small fruits 14 Tree fruits 15 Truck and garden crops 15 Field crops 17 Ornamental herbaceous garden plants 19 Ornamental shrubs and vines 19 Shade trees 20 Noneconomic plants 21 Feeding by grubs 22 Natural enemies of the beetle 24 Native enemies 24 Imported parasitic insects 24 Entomogenous diseases 26 Fungi 26 Protozoans 26 Nematodes 26 Rickettsias 27 Bacteria 3T Status of the beetle in 1962 ^ 29 GroHth Thnaih Afrloihiril Piainu This handbook supersedes Circular No. 332, General Information on the Japanese Beetle in the United States Washington, D.C. Issued Deceniber 1962 The in the UNITED STATES By WALTER E. FLEMING, Japanese Beetle and European Chafer Investigations, Entomology Research Division, Agricultural Research Service The Japanese beetle Pcpillia Japónica Newm., one of our plant pests of foreign origin, was first found in the United States in 1916 in a nursery near Riverton, N.J.