Gene Flow and Geographic Variation in Natural Populations of Alnus Acumi1'lata Ssp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cintia Luz.Pdf
Cíntia Luíza da Silva Luz Filogenia e sistemática de Schinus L. (Anacardiaceae), com revisão de um clado endêmico das matas nebulares andinas Phylogeny and systematics of Schinus L. (Anacardiaceae), with revision of a clade endemic to the Andean cloud forests Tese apresentada ao Instituto de Biociências da Universidade de São Paulo, para obtenção de Título de Doutor em Ciências, na Área de Botânica. Orientador: Dr. José Rubens Pirani São Paulo 2017 Luz, Cíntia Luíza da Silva Filogenia e sistemática de Schinus L. (Anacardiaceae), com revisão de um clado endêmico das matas nebulares andinas Número de páginas: 176 Tese (Doutorado) - Instituto de Biociências da Universidade de São Paulo. Departamento de Botânica. 1. Anacardiaceae 2. Schinus 3. Filogenia 4. Taxonomia vegetal I. Universidade de São Paulo. Instituto de Biociências. Departamento de Botânica Comissão julgadora: ______________________________ ______________________________ Prof(a). Dr.(a) Prof(a). Dr.(a) ______________________________ ______________________________ Prof(a). Dr.(a) Prof(a). Dr.(a) _____________________________________ Prof. Dr. José Rubens Pirani Orientador Ao Luciano Luz, pelo entusiasmo botânico, companheirismo e dedicação aos Schinus Esta é a estória. Ia um menino, com os tios, passar dias no lugar onde se construía a grande cidade. Era uma viagem inventada no feliz; para ele, produzia-se em caso de sonho. Saíam ainda com o escuro, o ar fino de cheiros desconhecidos. A mãe e o pai vinham trazê-lo ao aeroporto. A tia e o tio tomavam conta dele, justínhamente. Sorria-se, saudava-se, todos se ouviam e falavam. O avião era da companhia, especial, de quatro lugares. Respondiam-lhe a todas as perguntas, até o piloto conversou com ele. -

Global Survey of Ex Situ Betulaceae Collections Global Survey of Ex Situ Betulaceae Collections
Global Survey of Ex situ Betulaceae Collections Global Survey of Ex situ Betulaceae Collections By Emily Beech, Kirsty Shaw and Meirion Jones June 2015 Recommended citation: Beech, E., Shaw, K., & Jones, M. 2015. Global Survey of Ex situ Betulaceae Collections. BGCI. Acknowledgements BGCI gratefully acknowledges the many botanic gardens around the world that have contributed data to this survey (a full list of contributing gardens is provided in Annex 2). BGCI would also like to acknowledge the assistance of the following organisations in the promotion of the survey and the collection of data, including the Royal Botanic Gardens Edinburgh, Yorkshire Arboretum, University of Liverpool Ness Botanic Gardens, and Stone Lane Gardens & Arboretum (U.K.), and the Morton Arboretum (U.S.A). We would also like to thank contributors to The Red List of Betulaceae, which was a precursor to this ex situ survey. BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) BGCI is a membership organization linking botanic gardens is over 100 countries in a shared commitment to biodiversity conservation, sustainable use and environmental education. BGCI aims to mobilize botanic gardens and work with partners to secure plant diversity for the well-being of people and the planet. BGCI provides the Secretariat for the IUCN/SSC Global Tree Specialist Group. www.bgci.org FAUNA & FLORA INTERNATIONAL (FFI) FFI, founded in 1903 and the world’s oldest international conservation organization, acts to conserve threatened species and ecosystems worldwide, choosing solutions that are sustainable, based on sound science and take account of human needs. www.fauna-flora.org GLOBAL TREES CAMPAIGN (GTC) GTC is undertaken through a partnership between BGCI and FFI, working with a wide range of other organisations around the world, to save the world’s most threated trees and the habitats which they grow through the provision of information, delivery of conservation action and support for sustainable use. -

Origen Distribución Alnus Acuminata En El Mundo Biotecnología Al
ALISO Clasificación taxonómica Reino Plantae Subreino Tracheobionta División Magnoliophyta Clase Magnoliopsida Subclase Hamamelididae Orden Fagales Familia Betulaceae Género Alnus Especie acuminata Nombre binomial Alnus acuminata Kunth Subespecies Alnus acuminata ssp. Arguta http://fotos.miarroba.es/79496191/2-gira-chiquinquira-24-25-de-abril-126/in/1-agroforesteria/ Alnus acuminata ssp. Glabrata Alnus acuminata ssp. Acuminata Origen Distribución El género (Alnus) pertenece al Distribución Altitudinal: Sinónimos hemisferio norte, con 30 especies. Entre los 1500 y los 3500 m.s.n.m. Alnus arguta Alnus spachii La única especie en el mundo que Distribución geográfica en Betula arguta ha logrado bajar en forma silvestre Colombia: Alnus pringlei al sur es Alnus acuminata. Se encuentra en las 3 cordilleras. Alnus mirbellii Alnus ferruginea Los alisos procedentes de Distribución geográfica en el Alnus jorullensis Norteamérica fueron poblando Mundo: Alnus castaneifolia Sudamérica luego de la formación Se encuentra en áreas montañosas del “puente” montañoso desde México hasta Panamá y Nombres comunes por región (Centroamérica) que une a estos continúa bajando por los Andes dos subconscientes, ocurrida hace Eje cafetero y Antioquia: hasta el norte de Argentina. unos 3 millones de años. Aliso y Cerezo Nariño, Cauca y Huila: Alnus acuminata en el mundo Cerezo y Pino aliso. Cundinamarca y Boyacá: Abedul Otros nombres comunes Aile Alder Andelerne Aul Chaquiro Elite Fresno Huauyu Ilamo Jaúl Lambrán Llite Palo de águila Palo de lamo Rambrán Saino www.discoverlife.org/mp/20m?kind=Alnus+acuminata&guide=Neotropical_flora&flags=glean:&mobile=close Biotecnología al servicio de la biodiversidad ... 1 Vivero Terra Forestal S.A.S. DESCRIPCIÓN BOTÁNICA Árbol Tronco Corteza http://corpomail.corpoica.org.co http://corpomail.corpoica.org.co http://corpomail.corpoica.org.co/BACFILES/BACDIGITAL/56519/56519.p Altura máxima: 40 m El tronco es recto, con Caducifolio y monoico. -

Magnolia Zotictla (Magnolia Sect. Macrophylla, Magnoliaceae): a New Species from the Southern Sierra Madre Oriental, México
Phytotaxa 513 (4): 271–281 ISSN 1179-3155 (print edition) https://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2021 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.513.4.1 Magnolia zotictla (Magnolia sect. Macrophylla, Magnoliaceae): a new species from the southern Sierra Madre Oriental, México ARTURO SÁNCHEZ-GONZÁLEZ1,3, MARISOL GUTIÉRREZ-LOZANO1,4, REYNA DOMÍNGUEZ YESCAS2,5, ADRIANA GISELA HERNÁNDEZ-ÁLVAREZ1,6, A. SALOMÉ ORTEGA-PEÑA2,7 & J. ANTONIO VÁZQUEZ- GARCÍA2,8* 1 Universidad Autónoma del Estado de Hidalgo, Centro de Investigaciones Biológicas, Ciudad del Conocimiento, km. 4.5 carr. Pachuca- Tulancingo, Mineral de la Reforma, Hidalgo, 42184, México 2 Herbario IBUG, Instituto de Botánica, Departamento de Botánica y Zoología, Centro Universitario de Ciencias Biológicas y Agro- pecuarias, Universidad de Guadalajara, km. 15.5 carr. Guadalajara-Nogales, Las Agujas, Zapopan, Jalisco, 45221, México 3 [email protected]; https://orcid.org/0000-0002-3190-8789 4 [email protected]; https://orcid.org/0000-0002-4567-9761 5 [email protected]; https://orcid.org/0000-0002-4169-6871 6 [email protected]; https://orcid.org/0000-0002-9241-8513 7 [email protected]; https://orcid.org/0000-0002-3297-8316 8 [email protected]; https://orcid.org/0000-0002-8393-5906 *Corresponding author Abstract A new species of Magnolia from the southern Sierra Madre Oriental, Mexico, is described and illustrated, providing information about its habitat distribution, ecology, biogeography and conservation status. After 12 fieldwork expeditions near the border of the states of Hidalgo and Puebla, we have developed morphological, ecological and biogeographic data to support recognition of populations from Acaxochitlán, Hidalgo and Pahuatlán, Puebla as a distinct species of Magnolia sect. -
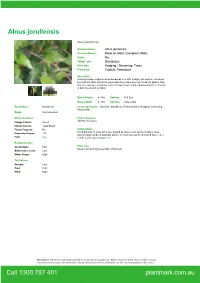
Alnus Jorullensis
Alnus jorullensis Alnus jorullensis Botanical Name: Alnus jorullensis Common Names: Mexican Alder, Evergreen Alder, Native: No Foliage Type: Deciduous Plant Type: Hedging / Screening, Trees Plant Habit: Conical, Pendulous Description: A fast growing evergreen-semi deciduous tree with a large root system. Loves wet feet and will often search for pipes and other water sources. Grows to approx 10m, this is a vigorous, fast grower with a broad crown and pendulous branches. Foliage is dark green and serrated. Mature Height: 4-10m Position: Full Sun Mature Width: 4-10m Soil Type: Clay, Loam Family Name: Betulaceae Landscape Use(s): Borders / Shrubbery, Formal Garden, Hedging / Screening, Wind Break Origin: SouthAmerica Characteristics: Pest & Diseases: Aphids, Possums Foliage Colours: Green Flower Colours: Insignificant Flower Fragrant: No Cultural Notes: Avoid planting to close to homes, paving as these roots can be invasive. Best Flowering Season: TBA position large gardens and park land or in flooded areas on farm land where they Fruit: Yes can be used a great shade tree. Requirements: Growth Rate: Fast Plant Care: Keep moist during dry periods, Mulch well Maintenance Level: Low Water Usage: High Tolerances: Drought: Low Frost: High Wind: High Disclaimer: Information and images provided is to be used as a guide only. While every reasonable effort is made to ensure accuracy and relevancy of all information, any decisions based on this information are the sole responsibility of the viewer. Call 1300 787 401 plantmark.com.au. -

The Mediterranean Palynological Societies Symposium 2019
The Mediterranean Palynological Societies Symposium 2019. Abstract book. Stéphanie Desprat, Anne-Laure Daniau, Maria Fernanda Sánchez Goñi To cite this version: Stéphanie Desprat, Anne-Laure Daniau, Maria Fernanda Sánchez Goñi. The Mediterranean Palyno- logical Societies Symposium 2019. Abstract book.. MedPalyno 2019, Jul 2019, Bordeaux, France. Université de Bordeaux, pp.142, 2019, 978-2-9562881-3-8. hal-02274992 HAL Id: hal-02274992 https://hal.archives-ouvertes.fr/hal-02274992 Submitted on 30 Aug 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. ABSTRACT BOOK The Mediterranean Palynological Societies Symposium 2019 The joint symposium of the APLF, APLE and GPP-SBI Bordeaux, July 9-10-11, 2019 Title: The Mediterranean Palynological Societies Symposium 2019. Abstract book. Editors: St´ephanie Desprat, Anne-Laure Daniau and Mar´ıa Fernanda S´anchez Go˜ni Publisher: Université de Bordeaux IBSN: 978-2-9562881-3-8 E-book available on https://hal.archives-ouvertes.fr/ ORGANIZING COMMITTEE Local committee from the EPOC research unit (UMR 5805: CNRS, Universite´ de Bordeaux, EPHE) Charlotte Clement´ Anne-Laure Daniau Stephanie´ Desprat Ludovic Devaux Tiffanie Fourcade Marion Genet Muriel Georget Laurent Londeix Maria F. Sanchez Goni˜ Coralie Zorzi Enlarged committee - Presidents of the APLF, GPPSBI and APLE Vincent Lebreton, HNHP, UMR 7194 CNRS-Mus´eumNational d’Histoire Naturelle (MNHN)-UPVD (France) Anna Maria Mercuri, Universit`adegli Studi di Modena e Reggio Emilia, Department of Life Sciences (Italy) Pilar S. -

2005 Meeting, La Jolla, CA
Where Cures Begin. Phytochemical Society of North America Welcome to this year’s annual meeting of the Phytochemical Society of North America and to the Salk Institute for Biological Studies. The theme of this year’s meeting, integrative plant biochemistry, is meant to celebrate the past accomplishments of the organization and its scientific focus, the growing importance of phytochemistry and plant biochemistry to the public, and to set a course for the future, linking the past with the present and attracting a wider breadth of scientists and disciplines to the society as we look to 2010 and beyond. This is a critical time in plant biochemistry as the tools available to us all provide unprecedented opportunities for discovery. However, we are increasingly faced with shrinking funds available for discovery-based research. Moreover, as a society, we share a collective responsibility for mentoring future plant biochemists and phytochemists who will lead us beyond 2010. We also must increase our participation in the political process to ensure that the next generation of scientists have available the funds to make the seminal discoveries that increase our fundamental understanding of how plants have evolved to survive and prosper in challenging ecosystems. These discoveries provide the necessary foundation for translating basic research into technology that will play critical roles in the health and well being of mankind. So while you all participate in the scientific discussions throughout the meeting, jot down ideas for future meetings, thoughts on how the society can evolve and play an increasingly critical role in advocating the economic importance of plant biochemistry for future generations and how the society can lead efforts to systemize and make available the exponentially expanding amount of data we are all accumulating. -

Descriptions of the Plant Types
APPENDIX A Descriptions of the plant types The plant life forms employed in the model are listed, with examples, in the main text (Table 2). They are described in this appendix in more detail, including environmental relations, physiognomic characters, prototypic and other characteristic taxa, and relevant literature. A list of the forms, with physiognomic characters, is included. Sources of vegetation data relevant to particular life forms are cited with the respective forms in the text of the appendix. General references, especially descriptions of regional vegetation, are listed by region at the end of the appendix. Plant form Plant size Leaf size Leaf (Stem) structure Trees (Broad-leaved) Evergreen I. Tropical Rainforest Trees (lowland. montane) tall, med. large-med. cor. 2. Tropical Evergreen Microphyll Trees medium small cor. 3. Tropical Evergreen Sclerophyll Trees med.-tall medium seier. 4. Temperate Broad-Evergreen Trees a. Warm-Temperate Evergreen med.-small med.-small seier. b. Mediterranean Evergreen med.-small small seier. c. Temperate Broad-Leaved Rainforest medium med.-Iarge scler. Deciduous 5. Raingreen Broad-Leaved Trees a. Monsoon mesomorphic (lowland. montane) medium med.-small mal. b. Woodland xeromorphic small-med. small mal. 6. Summergreen Broad-Leaved Trees a. typical-temperate mesophyllous medium medium mal. b. cool-summer microphyllous medium small mal. Trees (Narrow and needle-leaved) Evergreen 7. Tropical Linear-Leaved Trees tall-med. large cor. 8. Tropical Xeric Needle-Trees medium small-dwarf cor.-scler. 9. Temperate Rainforest Needle-Trees tall large-med. cor. 10. Temperate Needle-Leaved Trees a. Heliophilic Large-Needled medium large cor. b. Mediterranean med.-tall med.-dwarf cor.-scler. -

Micrdrilms International 300 N
A FUELWOOD MANAGEMENT PLAN AND COST- BENEFIT ANALYSIS FOR ALDER (ALNUS JORULLENSIS) IN THE ECUADORIAN ANDES Item Type text; Thesis-Reproduction (electronic) Authors Dunn, Walter Wheat, 1958- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 03/10/2021 18:36:36 Link to Item http://hdl.handle.net/10150/276547 INFORMATION TO USERS This reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. For blurred pages, a good image of the page can be found in the adjacent frame. -

FINAL REPORT BP CONSERVATION PROGRAMME Alder Amazon
FINAL REPORT BP CONSERVATION PROGRAMME Alder amazon–neglected issue in conservation priorities- defining its population status and distribution. Bronze Award 2003 Team leader: Luis Rivera Team member: Luciana Abendaño Team co-leader: Natalia Politi Team member: Guadalupe Peter E-mail: [email protected] 1 Alder amazon Project 2003 – BP CONSERVATION PROGRAMME SUMMARY This project is the first contribution to the knowledge of the species' population levels of Alder amazon (Amazona tucumana) throughout its distribution in Argentina. We recorded 5387 individuals in 12 of 18 localities, 7 are new localities for the species. Two areas concentrate 94% of the individuals recorded, one extends between El Nogalar and San Francisco and the other from Sierra (Sa.) de Santa Bárbara to El Rey National Park (NP), these two areas hold the last mature cloud forests remnants as well as continuous forested areas. Long term efforts for the conservation of this endemic species should prioritized on these areas by providing legal protection to remnant forests (e.g., basin-protection forests) and the promotion of the creation of privately-owned reserves (e.g., through tax exemptions). The largest Alder amazon population was detected in Sa. de Santa Bárbara, is not included in protected areas, and is seriously threatened by different human activities. Las Lancitas Provincial Reserve nearby should be extended towards the upper part of Sa. de Santa Bárbara to protect the breeding habitat of the species. The number of Alder amazons recorded represent less than the third part of those exported between 1985 and 1989 (18,641), what demonstrates that those captures had a strong effect on wild population levels; this information contributes to evaluate future trends of the species. -

Research Design and General Objectives
Forest quality in the southwest of Mexico City. Assessment towards ecological restoration of ecosystem services Thesis submitted in partial fulfilment of the requirements of the degree Doctor rer. nat. of the Faculty of Forest and Environmental Sciences, Albert-Ludwigs-Universität Freiburg im Breisgau, Germany By Víctor Ávila-Akerberg Freiburg im Breisgau, Germany 2009 Dean: Prof. Dr. Heinz Rennenberg First supervisor: Prof. Dr. Werner Konold Second supervisor: Prof. Dr. Albert Reif Date of disputation: December 9th 2009 Acknowledgements This thesis is dedicated to the forests in the area under study and to my family and friends! Thanks to Dr. Werner Konold, for accepting me as a PhD student, having trusted on my research, for always being there whenever I needed him, and for encouraging and supporting my trips to courses and conferences around the world, vielen Dank! I would like to thank Dr. Albert Reif for being my second supervisor and for the given advice and comments on the thesis. I would like to thank Dr. Lucia Almeida, for having taught me so many things, for believing in me and together having achieved so much in the Magdalena river watershed. My great appreciation goes to Dr. Jorge Meave del Castillo, for advising me and have shared part of his enormous experience and patience on scientific writing. Special thanks go to Esther Muschelknautz, for always being there to answer the administrative questions, attending and organizing the milestones and the extra- curricular courses in the International PhD Program “Forestry in transition”. During the last three years of my life, I have met and shared moments with many wonderful persons. -

Novel Asian Species, Worldwide Phylogeny and Evolutionary Relationships
fungal biology 120 (2016) 1554e1581 journal homepage: www.elsevier.com/locate/funbio Lactarius subgenus Russularia (Basidiomycota, Russulales): novel Asian species, worldwide phylogeny and evolutionary relationships Komsit WISITRASSAMEEWONGa,b,c,*, Brian P. LOONEYd, Huyen T. LEe, Eske DE CROPc, Kanad DASf, Kobeke VAN DE PUTTEc, Ursula EBERHARDTg, Guo JIAYUh,i, Dirk STUBBEj, Kevin D. HYDEa,b, Annemieke VERBEKENc, Jorinde NUYTINCKk aInstitute of Excellence in Fungal Research, Mae Fah Luang University, 333 Moo 1, Thasud Sub-district, Muang District, Chiang Rai 57100, Thailand bSchool of Science, Mae Fah Luang University, 333 Moo 1, Thasud Sub-district, Muang District, Chiang Rai 57100, Thailand cResearch Group Mycology, Department of Biology, Gent University, K.L. Ledeganckstraat 35, 9000 Gent, Belgium dDepartment of Ecology and Evolutionary Biology, University of Tennessee, 332 Hesler Biology Building, Knoxville, TN, USA eFaculty of Environment, Hanoi University of Natural Resources and Environment, 41A Phu Dien Road, Phu Dien Ward, North - Tu Liem District, Hanoi, Viet Nam fBotanical Survey of India, Cryptogamic Unit, P.O. Botanic Garden, Howrah 711103, India gStaatliches Museum fur€ Naturkunde Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany hCentre for Mountain Ecosystem Studies, Kunming Institute of Botany, Chinese Academy of Sciences, Lanhei 132, Heilongtan, Kunming 650201, Yunnan, China iWorld Agroforestry Centre, China & East-Asia Office, 132 Lanhei Road, Kunming 650201, China jService of Mycology and Aerobiology, BCCM/IHEM Fungal Collection, Scientific Institute of Public Health, Rue J. Wytsmanstraat 14, Brussels 1050, Belgium kNaturalis Biodiversity Center, P.O. Box 9517, 2300RA Leiden, The Netherlands article info abstract Article history: Lactarius subg. Russularia is a large group of milkcaps occurring almost worldwide and dom- Received 19 April 2016 inant in many ecosystems.