2005 Meeting, La Jolla, CA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proceedings Amurga Co
PROCEEDINGS OF THE AMURGA INTERNATIONAL CONFERENCES ON ISLAND BIODIVERSITY 2011 PROCEEDINGS OF THE AMURGA INTERNATIONAL CONFERENCES ON ISLAND BIODIVERSITY 2011 Coordination: Juli Caujapé-Castells Funded and edited by: Fundación Canaria Amurga Maspalomas Colaboration: Faro Media Cover design & layout: Estudio Creativo Javier Ojeda © Fundación Canaria Amurga Maspalomas Gran Canaria, December 2013 ISBN: 978-84-616-7394-0 How to cite this volume: Caujapé-Castells J, Nieto Feliner G, Fernández Palacios JM (eds.) (2013) Proceedings of the Amurga international conferences on island biodiversity 2011. Fundación Canaria Amurga-Maspalomas, Las Palmas de Gran Canaria, Spain. All rights reserved. Any unauthorized reprint or use of this material is prohibited. No part of this book may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system without express written permission from the author / publisher. SCIENTIFIC EDITORS Juli Caujapé-Castells Jardín Botánico Canario “Viera y Clavijo” - Unidad Asociada CSIC Consejería de Medio Ambiente y Emergencias, Cabildo de Gran Canaria Gonzalo Nieto Feliner Real Jardín Botánico de Madrid-CSIC José María Fernández Palacios Universidad de La Laguna SCIENTIFIC COMMITTEE Juli Caujapé-Castells, Gonzalo Nieto Feliner, David Bramwell, Águedo Marrero Rodríguez, Julia Pérez de Paz, Bernardo Navarro-Valdivielso, Ruth Jaén-Molina, Rosa Febles Hernández, Pablo Vargas. Isabel Sanmartín. ORGANIZING COMMITTEE Pedro -

The Evolutionary Dynamics of Genes and Genomes: Copy Number Variation of the Chalcone Synthase Gene in the Context of Brassicaceae Evolution
The Evolutionary Dynamics of Genes and Genomes: Copy Number Variation of the Chalcone Synthase Gene in the Context of Brassicaceae Evolution Dissertation submitted to the Combined Faculties for Natural Sciences and for Mathematics of the Ruperto-Carola University of Heidelberg, Germany for the degree of Doctor of Natural Sciences presented by Liza Paola Ding born in Mosbach, Baden-Württemberg, Germany Oral examination: 22.12.2014 Referees: Prof. Dr. Marcus A. Koch Prof. Dr. Claudia Erbar Table of contents INTRODUCTION ............................................................................................................. 18 1 THE MUSTARD FAMILY ....................................................................................... 19 2 THE TRIBAL SYSTEM OF THE BRASSICACEAE ........................................... 22 3 CHALCONE SYNTHASE ........................................................................................ 23 PART 1: TROUBLE WITH THE OUTGROUP............................................................ 27 4 MATERIAL AND METHODS ................................................................................. 28 4.1 Experimental set-up ......................................................................................................................... 28 4.1.1 Plant material and data composition .............................................................................................. 28 4.1.2 DNA extraction and PCR amplification ........................................................................................ -

Gene Flow and Geographic Variation in Natural Populations of Alnus Acumi1'lata Ssp
Rev. Biol. Trop., 47(4): 739-753, 1999 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Gene flow and geographic variation in natural populations of Alnus acumi1'lata ssp. arguta (Fagales: Betulaceae) in Costa Rica and Panama OIman Murillo) and Osear Roeha2 Instituto Tecnológico de Costa Rica, Escuela de Ing. Forestal.Apartado 159 7050 Cartago, Costa Rica. Fax: 591-4182. e-mail: omurillo@itcr.. ac.cr 2 Universidad de Costa Rica, Escuela de Biología, Campus San Pedro, San José, Costa Rica. e-mail: [email protected] Received 16-IX-1998. Corrected 05-IV-1999. Accepted 16-IV-1999 Abstract: Seventeen natural populations in Costa Rica andPanama were used to asses geneflow and geographic patternsof genetic variation in tbis tree species. Gene flow analysis was based on the methods of rare alleles and FST (Index of genetic similarity M), using the only four polymorphic gene loci among 22 investigated (PGI-B, PGM-A, MNR-A and IDH-A). The geographic variation analysiswas based on Pearson 's correlations between four geographic and 14 genetic variables. Sorne evidence of isolation by distance and a weak gene flow among geographic regions was found. Patterns of elinal variation in relation to altitude (r = -0.62 for genetic diversity) and latitude (r = -0.77 for PGI-B3) were also observed, supporting the hypothesis of isolation by distance. No privatealleles were found at the single population level. Key words: Alnus acuminata, isozymes, gene flow, eline, geographic variation, Costa Rica, Panarna. Pollen and seed moverrient among counteract any potential for genetic drift subdivided popuIations in a tree speeies, (Hamrick 1992 in Boshieret al. -

Global Survey of Ex Situ Betulaceae Collections Global Survey of Ex Situ Betulaceae Collections
Global Survey of Ex situ Betulaceae Collections Global Survey of Ex situ Betulaceae Collections By Emily Beech, Kirsty Shaw and Meirion Jones June 2015 Recommended citation: Beech, E., Shaw, K., & Jones, M. 2015. Global Survey of Ex situ Betulaceae Collections. BGCI. Acknowledgements BGCI gratefully acknowledges the many botanic gardens around the world that have contributed data to this survey (a full list of contributing gardens is provided in Annex 2). BGCI would also like to acknowledge the assistance of the following organisations in the promotion of the survey and the collection of data, including the Royal Botanic Gardens Edinburgh, Yorkshire Arboretum, University of Liverpool Ness Botanic Gardens, and Stone Lane Gardens & Arboretum (U.K.), and the Morton Arboretum (U.S.A). We would also like to thank contributors to The Red List of Betulaceae, which was a precursor to this ex situ survey. BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) BGCI is a membership organization linking botanic gardens is over 100 countries in a shared commitment to biodiversity conservation, sustainable use and environmental education. BGCI aims to mobilize botanic gardens and work with partners to secure plant diversity for the well-being of people and the planet. BGCI provides the Secretariat for the IUCN/SSC Global Tree Specialist Group. www.bgci.org FAUNA & FLORA INTERNATIONAL (FFI) FFI, founded in 1903 and the world’s oldest international conservation organization, acts to conserve threatened species and ecosystems worldwide, choosing solutions that are sustainable, based on sound science and take account of human needs. www.fauna-flora.org GLOBAL TREES CAMPAIGN (GTC) GTC is undertaken through a partnership between BGCI and FFI, working with a wide range of other organisations around the world, to save the world’s most threated trees and the habitats which they grow through the provision of information, delivery of conservation action and support for sustainable use. -

Phoenix Active Management Area Low-Water-Use/Drought-Tolerant Plant List
Arizona Department of Water Resources Phoenix Active Management Area Low-Water-Use/Drought-Tolerant Plant List Official Regulatory List for the Phoenix Active Management Area Fourth Management Plan Arizona Department of Water Resources 1110 West Washington St. Ste. 310 Phoenix, AZ 85007 www.azwater.gov 602-771-8585 Phoenix Active Management Area Low-Water-Use/Drought-Tolerant Plant List Acknowledgements The Phoenix AMA list was prepared in 2004 by the Arizona Department of Water Resources (ADWR) in cooperation with the Landscape Technical Advisory Committee of the Arizona Municipal Water Users Association, comprised of experts from the Desert Botanical Garden, the Arizona Department of Transporation and various municipal, nursery and landscape specialists. ADWR extends its gratitude to the following members of the Plant List Advisory Committee for their generous contribution of time and expertise: Rita Jo Anthony, Wild Seed Judy Mielke, Logan Simpson Design John Augustine, Desert Tree Farm Terry Mikel, U of A Cooperative Extension Robyn Baker, City of Scottsdale Jo Miller, City of Glendale Louisa Ballard, ASU Arboritum Ron Moody, Dixileta Gardens Mike Barry, City of Chandler Ed Mulrean, Arid Zone Trees Richard Bond, City of Tempe Kent Newland, City of Phoenix Donna Difrancesco, City of Mesa Steve Priebe, City of Phornix Joe Ewan, Arizona State University Janet Rademacher, Mountain States Nursery Judy Gausman, AZ Landscape Contractors Assn. Rick Templeton, City of Phoenix Glenn Fahringer, Earth Care Cathy Rymer, Town of Gilbert Cheryl Goar, Arizona Nurssery Assn. Jeff Sargent, City of Peoria Mary Irish, Garden writer Mark Schalliol, ADOT Matt Johnson, U of A Desert Legum Christy Ten Eyck, Ten Eyck Landscape Architects Jeff Lee, City of Mesa Gordon Wahl, ADWR Kirti Mathura, Desert Botanical Garden Karen Young, Town of Gilbert Cover Photo: Blooming Teddy bear cholla (Cylindropuntia bigelovii) at Organ Pipe Cactus National Monutment. -

Fatty Acid Composition of Matthiola Longipetala Ssp. Bicornis from Turkey
INTERNATİONAL JOURNAL OF AGRİCULTURE & BİOLOGY ISSN Print: 1560–8530; ISSN Online: 1814–9596 10–140/DNK/2011/13–4–581–585 http://www.fspublishers.org Full Length Article Fatty Acid Composition of Matthiola longipetala ssp. Bicornis from Turkey SENGUL KARAMAN1, MEHMET GULSEVEN, NAZAN COMLEKCIOGLU AND AHMET ILCIM Department of Biology, Faculty of Science and Arts, Kahramanmaras Sutcu Imam University, Avsar Campus, 46000 Kahramanmaras, Turkey 1Corresponding author’s e-mail: [email protected] ABSTRACT The fatty acid composition and morphological characteristics of Matthiola longipetala ssp. bicornis was studied. Wild Matthiola plants and seeds were collected from its native areas in Turkey. The native plants were cultivated in two different sowing times (autumn sowing & late winter sowing) and some morphological characters and seed oil composition were measured. Plant height, number of siliqua, siliqua length, seed yield, thousand seeds weight and seed length values were found to be the highest in autumn sowings. The quantative analysis of seed oil of cultures and wild plants were compared and oil ratio were obtained as 21, 11 and 11% in autumn sowing, late winter sowing cultures and native plants respectively. γ- linolenic acid was found as main component at all Matthiola plants. The ratio of γ-linolenic acid was obtained 65.6, 63.0 and 62.6% in autumn sowing, late winter sowing and wild plants, respectively. The other main constituents of the all oils were obtained as oleic and linoleic acids. The ratios of oleic and linoleic acids were obtained 11.04% and 10.70% in autumn sowing, 13.18% and 11.53% in late winter sowing, 12.46% and 11.01% in wild plants, respectively. -
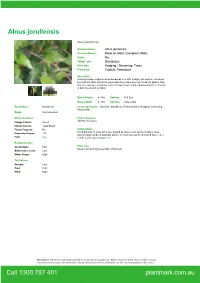
Alnus Jorullensis
Alnus jorullensis Alnus jorullensis Botanical Name: Alnus jorullensis Common Names: Mexican Alder, Evergreen Alder, Native: No Foliage Type: Deciduous Plant Type: Hedging / Screening, Trees Plant Habit: Conical, Pendulous Description: A fast growing evergreen-semi deciduous tree with a large root system. Loves wet feet and will often search for pipes and other water sources. Grows to approx 10m, this is a vigorous, fast grower with a broad crown and pendulous branches. Foliage is dark green and serrated. Mature Height: 4-10m Position: Full Sun Mature Width: 4-10m Soil Type: Clay, Loam Family Name: Betulaceae Landscape Use(s): Borders / Shrubbery, Formal Garden, Hedging / Screening, Wind Break Origin: SouthAmerica Characteristics: Pest & Diseases: Aphids, Possums Foliage Colours: Green Flower Colours: Insignificant Flower Fragrant: No Cultural Notes: Avoid planting to close to homes, paving as these roots can be invasive. Best Flowering Season: TBA position large gardens and park land or in flooded areas on farm land where they Fruit: Yes can be used a great shade tree. Requirements: Growth Rate: Fast Plant Care: Keep moist during dry periods, Mulch well Maintenance Level: Low Water Usage: High Tolerances: Drought: Low Frost: High Wind: High Disclaimer: Information and images provided is to be used as a guide only. While every reasonable effort is made to ensure accuracy and relevancy of all information, any decisions based on this information are the sole responsibility of the viewer. Call 1300 787 401 plantmark.com.au. -

London Rocket Tech Bulletin – ND
4/6/2020 London Rocket London Rocket Sisymbrium irio L. Family: Brassicaceae. Names: Sisymbrium was the Greek name of a fragrant herb. London Rocket. Summary: An erect, annual, many branched plant, with deeply lobed leaves that does not form a rosette. It has clusters of small, 4-petalled, yellow flowers in late winter to spring on the tops of stems that form long (25-110 mm), narrow seed pods that may be slightly curved. Description: Cotyledons: Two. Club shaped, Tip rounded. Sides convex. Base tapered. Surface hairless. Petiole longer than the blade. First Leaves: Club shaped, paired. The first pair have rounded tips and smooth edges. The second pair have pointed tips and toothed edges. Hairless or a few hairs. Leaves: Alternate. Does not form a rosette. Stipules - None. Petiole - On lower leaves. Blade - 30-160 mm long x 13-70 mm wide, triangular in outline, deeply lobed or serrated or toothed (usually 2-6 pairs), lobes are usually toothed, end lobe is pointed and larger than the side lobes. The side lobes usually point towards the base of the leaf. Tip pointed. Smooth and hairless or a few scattered hairs. Stem leaves - Alternate. Similar to rosette leaves but not as lobed or unlobed, sometimes arrow shaped. Hairless or small hairs. Stems: Slender, erect, round, up to 1000 mm tall. Often with slender, curved, simple hairs near the base, usually hairless near the top. Usually much branched from the base with spreading stems. Flower head: www.herbiguide.com.au/Descriptions/hg_London_Rocket.htm 1/8 4/6/2020 London Rocket Flowers are in clusters at the top of the stem which then elongates as the fruits mature underneath. -

GARDENERGARDENER® Thethe Magazinemagazine Ofof Thethe Aamericanmerican Horticulturalhorticultural Societysociety July / August 2007
TheThe AmericanAmerican GARDENERGARDENER® TheThe MagazineMagazine ofof thethe AAmericanmerican HorticulturalHorticultural SocietySociety July / August 2007 pleasures of the Evening Garden HardyHardy PlantsPlants forfor Cold-ClimateCold-Climate RegionsRegions EveningEvening PrimrosesPrimroses DesigningDesigning withwith See-ThroughSee-Through PlantsPlants WIN THE BATTLE OF THE BULB The OXO GOOD GRIPS Quick-Release Bulb Planter features a heavy gauge steel shaft with a soft, comfortable, non-slip handle, large enough to accommodate two hands. The Planter’s patented Quick-Release lever replaces soil with a quick and easy squeeze. Dig in! 1.800.545.4411 www.oxo.com contents Volume 86, Number 4 . July / August 2007 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 7 NEWS FROM AHS AHS award winners honored, President’s Council trip to Charlotte, fall plant and antiques sale at River Farm, America in Bloom Symposium in Arkansas, Eagle Scout project enhances River Farm garden, second AHS page 7 online plant seminar on annuals a success, page 39 Homestead in the Garden Weekend. 14 AHS PARTNERS IN PROFILE YourOutDoors, Inc. 16 PLEASURES OF THE EVENING GARDEN BY PETER LOEWER 44 ONE ON ONE WITH… Enjoy the garden after dark with appropriate design, good lighting, and the addition of fragrant, night-blooming plants. Steve Martino, landscape architect. 46 NATURAL CONNECTIONS 22 THE LEGEND OF HIDDEN Parasitic dodder. HOLLOW BY BOB HILL GARDENER’S NOTEBOOK Working beneath the radar, 48 Harald Neubauer is one of the Groundcovers that control weeds, meadow rues suited for northern gardens, new propagation wizards who online seed and fruit identification guide, keeps wholesale and retail national “Call Before You Dig” number nurseries stocked with the lat- established, saving wild magnolias, Union est woody plant selections. -

CHEMICAL CONSTITUENTS of MATTHIOLA LONGIPETALA (Ssp
Journal de la Société Chimique de Tunisie, 2009, 11, 159-162 159 62&,(7(&+,0,48('(781,6,( CHEMICAL CONSTITUENTS OF MATTHIOLA LONGIPETALA (ssp. LIVIDA ) (Del.) MAIRE (BRASSICACEAE) GROWING IN TUNISIA SaoussenSaoussen Hammami, Hammami Ines Khoja, Nouha Nejia Mrad, Hichem Ben Jannet, M’hamed Ali Hamza, Zine Mighri * et al. Laboratoire de Chimie des Substances Naturelles et de Synthèse Organique (99/UR/12-26) Faculté des Sciences de Monastir, 5019 Monastir, Tunisia (Reçu le 31 Juillet 2009, accepté le 12 Octobre 2009) ABSTRACT: Investigation of methanolic extract from Matthiola longipetala (ssp. livida) (Del.) Maire Brassicaceae of Tunisian origin, led to the isolation of methylfirulate-4’-O-β-D glycoside 1 together with 2-O-methyl α-D- fructofuranose tetraacetate 2 as well as α-D-glycopyranose pentaacetate 3 and β-D glycopyranose pentaacetate 4, all structures were established using 1 and 2D NMR experiments, carbohydrates were identified via their acetylated derivatives. Key words: Matthiola longipetala (ssp. livida) (Del.) Maire, Brassicaceae, 4’-O-β-D-glycopyranosyl-methylfirulate 1,2-O-methyl α -D-fructofuranose tetraacetate 2, α and β-D-glycopyranoses pentaacetates 3 and 4. RESUME: le 4’-O-β-D-glycosylfirulate de méthyle 1 a été identifié pour la première fois dans l’extrait méthanolique de l’espèce Matthiola longipetala (ssp. livida) (Del.) Maire (Brassicaceae) poussant en Tunisie, le 2-O-méthyl-α-D- fructofuranose, l’α et le β-D-glycopyranose, ont été identifiés dans le même extrait via leurs dérivés acétylés 2, 3 et 4. Mots clés : Matthiola longipetala (ssp. livida) (Del.) Maire, Brassicaceae, 4’-O-β-D-glycosylfirulate de méthyle 1,2-O- méthyl-α-D-fructofuranose tétraacétate 2, l’α et le β-D-glycopyranoses petaacétylés 3 et 4. -

Phoenix AMA LWUPL
Arizona Department of Water Resources Phoenix Active Management Area Low-Water-Use/Drought-Tolerant Plant List Official Regulatory List for the Phoenix Active Management Area Fourth Management Plan Arizona Department of Water Resources 1110 West Washington St. Ste. 310 Phoenix, AZ 85007 www.azwater.gov 602-771-8585 Phoenix Active Management Area Low-Water-Use/Drought-Tolerant Plant List Acknowledgements The Phoenix AMA list was prepared in 2004 by the Arizona Department of Water Resources (ADWR) in cooperation with the Landscape Technical Advisory Committee of the Arizona Municipal Water Users Association, comprised of experts from the Desert Botanical Garden, the Arizona Department of Transporation and various municipal, nursery and landscape specialists. ADWR extends its gratitude to the following members of the Plant List Advisory Committee for their generous contribution of time and expertise: Rita Jo Anthony, Wild Seed Judy Mielke, Logan Simpson Design John Augustine, Desert Tree Farm Terry Mikel, U of A Cooperative Extension Robyn Baker, City of Scottsdale Jo Miller, City of Glendale Louisa Ballard, ASU Arboritum Ron Moody, Dixileta Gardens Mike Barry, City of Chandler Ed Mulrean, Arid Zone Trees Richard Bond, City of Tempe Kent Newland, City of Phoenix Donna Difrancesco, City of Mesa Steve Priebe, City of Phornix Joe Ewan, Arizona State University Janet Rademacher, Mountain States Nursery Judy Gausman, AZ Landscape Contractors Assn. Rick Templeton, City of Phoenix Glenn Fahringer, Earth Care Cathy Rymer, Town of Gilbert Cheryl Goar, Arizona Nurssery Assn. Jeff Sargent, City of Peoria Mary Irish, Garden writer Mark Schalliol, ADOT Matt Johnson, U of A Desert Legum Christy Ten Eyck, Ten Eyck Landscape Architects Jeff Lee, City of Mesa Gordon Wahl, ADWR Kirti Mathura, Desert Botanical Garden Karen Young, Town of Gilbert Cover Photo: Blooming Teddy bear cholla (Cylindropuntia bigelovii) at Organ Pipe Cactus National Monutment. -

Descriptions of the Plant Types
APPENDIX A Descriptions of the plant types The plant life forms employed in the model are listed, with examples, in the main text (Table 2). They are described in this appendix in more detail, including environmental relations, physiognomic characters, prototypic and other characteristic taxa, and relevant literature. A list of the forms, with physiognomic characters, is included. Sources of vegetation data relevant to particular life forms are cited with the respective forms in the text of the appendix. General references, especially descriptions of regional vegetation, are listed by region at the end of the appendix. Plant form Plant size Leaf size Leaf (Stem) structure Trees (Broad-leaved) Evergreen I. Tropical Rainforest Trees (lowland. montane) tall, med. large-med. cor. 2. Tropical Evergreen Microphyll Trees medium small cor. 3. Tropical Evergreen Sclerophyll Trees med.-tall medium seier. 4. Temperate Broad-Evergreen Trees a. Warm-Temperate Evergreen med.-small med.-small seier. b. Mediterranean Evergreen med.-small small seier. c. Temperate Broad-Leaved Rainforest medium med.-Iarge scler. Deciduous 5. Raingreen Broad-Leaved Trees a. Monsoon mesomorphic (lowland. montane) medium med.-small mal. b. Woodland xeromorphic small-med. small mal. 6. Summergreen Broad-Leaved Trees a. typical-temperate mesophyllous medium medium mal. b. cool-summer microphyllous medium small mal. Trees (Narrow and needle-leaved) Evergreen 7. Tropical Linear-Leaved Trees tall-med. large cor. 8. Tropical Xeric Needle-Trees medium small-dwarf cor.-scler. 9. Temperate Rainforest Needle-Trees tall large-med. cor. 10. Temperate Needle-Leaved Trees a. Heliophilic Large-Needled medium large cor. b. Mediterranean med.-tall med.-dwarf cor.-scler.