WO 2015/084633 Al 11 June 2015 (11.06.2015) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Regulation of Plant Monoterpene Biosynthesis in Relation to Fragrance
Molecular Regulation of Plant Monoterpene Biosynthesis In Relation To Fragrance Mazen K. El Tamer Promotor: Prof. Dr. A.G.J Voragen, hoogleraar in de Levensmiddelenchemie, Wageningen Universiteit Co-promotoren: Dr. ir. H.J Bouwmeester, senior onderzoeker, Business Unit Celcybernetica, Plant Research International Dr. ir. J.P Roozen, departement Agrotechnologie en Voedingswetenschappen, Wageningen Universiteit Promotiecommissie: Dr. M.C.R Franssen, Wageningen Universiteit Prof. Dr. J.H.A Kroeze, Wageningen Universiteit Prof. Dr. A.J van Tunen, Swammerdam Institute for Life Sciences, Universiteit van Amsterdam. Prof. Dr. R.G.F Visser, Wageningen Universiteit Mazen K. El Tamer Molecular Regulation Of Plant Monoterpene Biosynthesis In Relation To Fragrance Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, Prof. dr. ir. L. Speelman, in het openbaar te verdedigen op woensdag 27 november 2002 des namiddags te vier uur in de Aula Mazen K. El Tamer Molecular Regulation Of Plant Monoterpene Biosynthesis In Relation To Fragrance Proefschrift Wageningen Universiteit ISBN 90-5808-752-2 Cover and Invitation Design: Zeina K. El Tamer This thesis is dedicated to my Family & Friends Contents Abbreviations Chapter 1 General introduction and scope of the thesis 1 Chapter 2 Monoterpene biosynthesis in lemon (Citrus limon) cDNA isolation 21 and functional analysis of four monoterpene synthases Chapter 3 Domain swapping of Citrus limon monoterpene synthases: Impact 57 on enzymatic activity and -

Key Enzymes Involved in the Synthesis of Hops Phytochemical Compounds: from Structure, Functions to Applications
International Journal of Molecular Sciences Review Key Enzymes Involved in the Synthesis of Hops Phytochemical Compounds: From Structure, Functions to Applications Kai Hong , Limin Wang, Agbaka Johnpaul , Chenyan Lv * and Changwei Ma * College of Food Science and Nutritional Engineering, China Agricultural University, 17 Qinghua Donglu Road, Haidian District, Beijing 100083, China; [email protected] (K.H.); [email protected] (L.W.); [email protected] (A.J.) * Correspondence: [email protected] (C.L.); [email protected] (C.M.); Tel./Fax: +86-10-62737643 (C.M.) Abstract: Humulus lupulus L. is an essential source of aroma compounds, hop bitter acids, and xanthohumol derivatives mainly exploited as flavourings in beer brewing and with demonstrated potential for the treatment of certain diseases. To acquire a comprehensive understanding of the biosynthesis of these compounds, the primary enzymes involved in the three major pathways of hops’ phytochemical composition are herein critically summarized. Hops’ phytochemical components impart bitterness, aroma, and antioxidant activity to beers. The biosynthesis pathways have been extensively studied and enzymes play essential roles in the processes. Here, we introduced the enzymes involved in the biosynthesis of hop bitter acids, monoterpenes and xanthohumol deriva- tives, including the branched-chain aminotransferase (BCAT), branched-chain keto-acid dehydroge- nase (BCKDH), carboxyl CoA ligase (CCL), valerophenone synthase (VPS), prenyltransferase (PT), 1-deoxyxylulose-5-phosphate synthase (DXS), 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR), Geranyl diphosphate synthase (GPPS), monoterpene synthase enzymes (MTS), cinnamate Citation: Hong, K.; Wang, L.; 4-hydroxylase (C4H), chalcone synthase (CHS_H1), chalcone isomerase (CHI)-like proteins (CHIL), Johnpaul, A.; Lv, C.; Ma, C. -

Reduction by the Methylreductase System in Methanobacterium Bryantii WILLIAM B
JOURNAL OF BACTERIOLOGY, Jan. 1987, p. 87-92 Vol. 169, No. 1 0021-9193/87/010087-06$02.00/0 Copyright © 1987, American Society for Microbiology Inhibition by Corrins of the ATP-Dependent Activation and CO2 Reduction by the Methylreductase System in Methanobacterium bryantii WILLIAM B. WHITMAN'* AND RALPH S. WOLFE2 Department of Microbiology, University of Georgia, Athens, Georgia 30602,1 and Department of Microbiology, University ofIllinois, Urbana, Illinois 618012 Received 1 August 1986/Accepted 28 September 1986 Corrins inhibited the ATP-dependent activation of the methylreductase system and the methyl coenzyme M-dependent reduction of CO2 in extracts of Methanobacterium bryantii resolved from low-molecular-weight factors. The concentrations of cobinamides and cobamides required for one-half of maximal inhibition of the ATP-depen4ent activation were between 1 and 5 ,M. Cobinamides were more inhibitory at lower concentra- tiops than cobamides. Deoxyadenosylcobalamin was not inhibitory at concentrations up to 25 ,uM. The inhibition of CO2 reduction was competitive with respect to CO2. The concentration of methylcobalamin required for one-half of maximal inhibition was 5 ,M. Other cobamideg inhibited at similar concentrations, but diaquacobinami4e inhibited at lower concentrations. With respect to their affinities and specificities for corrins, inhibition of both the ATP-dependent activation'and CO2 reduction closely resembled the corrin- dependent activation of the methylreductase described in similar extracts (W. B. Whitman and R. S. Wolfe, J. Bacteriol. 164:165-172, 1985). However, whether the multiple effects of corrins are due to action at a single site is unknown. The effect of corrins (cobamides and cobinamides) on in CO2 reduction. -

Multi-Substrate Terpene Synthases: Their Occurrence and Physiological
FOCUSED REVIEW published: 12 July 2016 doi: 10.3389/fpls.2016.01019 Multi-Substrate Terpene Synthases: Edited by: Joshua L. Heazlewood, The University of Melbourne, Australia Their Occurrence and Physiological Reviewed by: Maaria Rosenkranz, Significance Helmholtz Zentrum München, Germany Leila Pazouki 1* and Ülo Niinemets 1, 2* Sandra Irmisch, University of British Columbia (UBC), 1 Department of Plant Physiology, Institute of Agricultural and Environmental Sciences, Estonian University of Life Sciences, Canada Tartu, Estonia, 2 Estonian Academy of Sciences, Tallinn, Estonia Pengxiang Fan, Michigan State University, USA Terpene synthases are responsible for synthesis of a large number of terpenes *Correspondence: in plants using substrates provided by two distinct metabolic pathways, the mevalonate-dependent pathway that is located in cytosol and has been suggested to be responsible for synthesis of sesquiterpenes (C15), and 2-C-methyl-D-erythritol-4-phosphate pathway located in plastids and suggested to be responsible for the synthesis of hemi- (C5), mono- (C10), and diterpenes (C20). Leila Pazouki did her undergraduate Recent advances in characterization of genes and enzymes responsible for substrate and degree at the University of Tehran and master degree in Plant Biotechnology end product biosynthesis as well as efforts in metabolic engineering have demonstrated at the University of Bu Ali Sina in Iran. existence of a number of multi-substrate terpene synthases. This review summarizes the She worked as a researcher at the progress in the characterization of such multi-substrate terpene synthases and suggests Agricultural Biotechnology Research Institute of Iran (ABRII) for four years. that the presence of multi-substrate use might have been significantly underestimated. -
The Wolfe Cycle Comes Full Circle
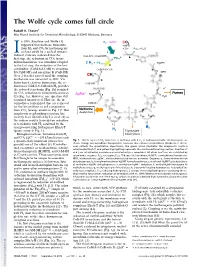
The Wolfe cycle comes full circle Rudolf K. Thauer1 Max Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany n 1988, Rouvière and Wolfe (1) H - ΔμNa+ 2 CO2 suggested that methane formation + MFR from H and CO by methanogenic + 2H+ *Fd + H O I 2 2 ox 2 archaea could be a cyclical process. j O = Indirect evidence indicated that the CoB-SH + CoM-SH fi *Fd 2- a rst step, the reduction of CO2 to for- red R mylmethanofuran, was somehow coupled + * H MPT 2 H2 Fdox 4 to the last step, the reduction of the het- h erodisulfide (CoM-S-S-CoB) to coenzyme CoM-S-S-CoB b MFR M (CoM-SH) and coenzyme B (CoB-SH). H Over 2 decades passed until the coupling C 4 10 mechanism was unraveled in 2011: Via g flavin-based electron bifurcation, the re- CoB-SH duction of CoM-S-S-CoB with H provides 2 H+ the reduced ferredoxin (Fig. 1h) required c + Purines for CO2 reduction to formylmethanofuran ΔμNa + H MPT 4 f H O (2) (Fig. 1a). However, one question still 2 remained unanswered: How are the in- termediates replenished that are removed CoM-SH for the biosynthesis of cell components H Methionine d from CO2 (orange arrows in Fig. 1)? This Acetyl-CoA e anaplerotic (replenishing) reaction has F420 F420H2 recently been identified by Lie et al. (3) as F420 F420H2 the sodium motive force-driven reduction H i of ferredoxin with H2 catalyzed by the i energy-converting hydrogenase EhaA-T H2 (green arrow in Fig. -

Annotation Guidelines for Experimental Procedures
Annotation Guidelines for Experimental Procedures Developed By Mohammed Alliheedi Robert Mercer Version 1 April 14th, 2018 1- Introduction and background information What is rhetorical move? A rhetorical move can be defined as a text fragment that conveys a distinct communicative goal, in other words, a sentence that implies an author’s specific purpose to readers. What are the types of rhetorical moves? There are several types of rhetorical moves. However, we are interested in 4 rhetorical moves that are common in the method section of a scientific article that follows the Introduction Methods Results and Discussion (IMRaD) structure. 1- Description of a method: It is concerned with a sentence(s) that describes experimental events (e.g., “Beads with bound proteins were washed six times (for 10 min under rotation at 4°C) with pulldown buffer and proteins harvested in SDS-sample buffer, separated by SDS-PAGE, and analyzed by autoradiography.” (Ester & Uetz, 2008)). 2- Appeal to authority: It is concerned with a sentence(s) that discusses the use of standard methods, protocols, and procedures. There are two types of this move: - A reference to a well-established “standard” method (e.g., the use of a method like “PCR” or “electrophoresis”). - A reference to a method that was previously described in the literature (e.g., “Protein was determined using fluorescamine assay [41].” (Larsen, Frandesn and Treiman, 2001)). 3- Source of materials: It is concerned with a sentence(s) that lists the source of biological materials that are used in the experiment (e.g., “All microalgal strains used in this study are available at the Elizabeth Aidar Microalgae Culture Collection, Department of Marine Biology, Federal Fluminense University, Brazil.” (Larsen, Frandesn and Treiman, 2001)). -

Untargetted Metabolomic Exploration of the Mycobacterium Tuberculosis Stress Response to Cinnamon Essential Oil
biomolecules Article Untargetted Metabolomic Exploration of the Mycobacterium tuberculosis Stress Response to Cinnamon Essential Oil Elwira Sieniawska 1,* , Rafał Sawicki 2 , Joanna Golus 2 and Milen I. Georgiev 3,4 1 Chair and Department of Pharmacognosy, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland 2 Chair and Department of Biochemistry and Biotechnology, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland; [email protected] (R.S.); [email protected] (J.G.) 3 Group of Plant Cell Biotechnology and Metabolomics, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, 139 Ruski Blvd., 4000 Plovdiv, Bulgaria; [email protected] 4 Center of Plant Systems Biology and Biotechnology, 4000 Plovdiv, Bulgaria * Correspondence: [email protected] Received: 6 January 2020; Accepted: 24 February 2020; Published: 26 February 2020 Abstract: The antimycobacterial activity of cinnamaldehyde has already been proven for laboratory strains and for clinical isolates. What is more, cinnamaldehyde was shown to threaten the mycobacterial plasma membrane integrity and to activate the stress response system. Following promising applications of metabolomics in drug discovery and development we aimed to explore the mycobacteria response to cinnamaldehyde within cinnamon essential oil treatment by untargeted liquid chromatography–mass spectrometry. The use of predictive metabolite pathway analysis and description of produced lipids enabled the evaluation of the stress symptoms shown by bacteria. This study suggests that bacteria exposed to cinnamaldehyde could reorganize their outer membrane as a physical barrier against stress factors. They probably lowered cell wall permeability and inner membrane fluidity, and possibly redirected carbon flow to store energy in triacylglycerols. Being a reactive compound, cinnamaldehyde may also contribute to disturbances in bacteria redox homeostasis and detoxification mechanisms. -

Floral Volatile Alleles Can Contribute to Pollinatormediated
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2014 Floral volatile alleles can contribute to pollinator-mediated reproductive isolation in monkeyflowers (Mimulus) Byers, Kelsey J R P ; Vela, James P ; Peng, Foen ; Riffell, Jeffrey A ; Bradshaw, HD Abstract: Pollinator-mediated reproductive isolation is a major factor in driving the diversification of flowering plants. Studies of floral traits involved in reproductive isolation have focused nearly exclusively on visual signals, such as flower color. The role of less obvious signals, such as floral scent, hasbeen studied only recently. In particular, the genetics of floral volatiles involved in mediating differential pollinator visitation remains unknown. The bumblebee-pollinated Mimulus lewisii and hummingbird- pollinated M. cardinalis are a model system for studying reproductive isolation via pollinator preference. We have shown that these two species differ in three floral terpenoid volatiles - D-limonene, -myrcene, and E--ocimene - that are attractive to bumblebee pollinators. By genetic mapping and in vitro enzyme activity analysis we demonstrate that these interspecific differences are consistent with allelic variation at two loci – LIMONENE-MYRCENE SYNTHASE (LMS) and OCIMENE SYNTHASE (OS). M. lewisii LMS (MlLMS) and OS (MlOS) are expressed most strongly in floral tissue in the last stages of floral development. M. cardinalis LMS (McLMS) is weakly expressed and has a nonsense mutation in exon 3. M. cardinalis OS (McOS) is expressed similarly to MlOS, but the encoded McOS enzyme produces no E--ocimene. Recapitulating the M. cardinalis phenotype by reducing the expression of MlLMS by RNAi in transgenic M. -

{Replace with the Title of Your Dissertation}
Metabolic Regulation and Genetic Tools for Bacterial Neutral Lipid Production A THESIS SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Nagendra Prasad Palani IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE Dr. Brett M. Barney September 2011 © Nagendra Prasad Palani 2011 Acknowledgements I express my most sincere thanks to Dr. Brett Barney. He has been an exceptional teacher, advisor and mentor for me. He has been unfailingly supportive during my time here and I cannot thank him enough for all he has taught me. I also thank the Department of Bioproducts & Biosystems Engineering, University of Minnesota, the National Science Foundation and the Department of Energy for their support of research in Dr. Barney’s lab. I thank my committee members Dr. Igor Libourel and Dr. Simo Sarkanen for posing critical questions about my research, helping me think better in the process. Their wisdom and insight often set me into thinking about my research from new perspectives. I thank fellow graduate and undergraduate students of the Barney lab and members of the Libourel lab for the many favors I have asked of them. I thank my friends here in the US for their fantastic company. I cannot express enough gratitude to my friends back home, who stepped in as sons and brothers whenever my family needed me. I thank my parents and my sister for their unwavering love, support and confidence in me. For her incredible patience and steadfast support, many thanks to my best friend and soon-to-be wife, Ajeetha. -

Rearrangement of Allylic Alcohols Herbert Barbehenn
Rochester Institute of Technology RIT Scholar Works Theses Thesis/Dissertation Collections 1-1-1971 Rearrangement of allylic alcohols Herbert Barbehenn Follow this and additional works at: http://scholarworks.rit.edu/theses Recommended Citation Barbehenn, Herbert, "Rearrangement of allylic alcohols" (1971). Thesis. Rochester Institute of Technology. Accessed from This Thesis is brought to you for free and open access by the Thesis/Dissertation Collections at RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. REARRANGEMENT OF ALLYLIC ALCOHOLS HERBERT S. BARBEHENN JANUARY, 1971 THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE APPROVED: Dr. Jerry Adduci Project Adviser Department Head Library Rochester Institute of Technology Rochester, New York To Rath, my wife - - - for the many lonely nights, for the many unfinished chores and for being herself. Acknowledgements Grateful appreciation is tendered to the many faculty members with whom it has been my pleasure to be associated with during the past eleven years at Rochester Institute of Technology. Special thanks are expressed to Dr. Jerry Adduci for his guidance and patience in seeing this endeavor to its conclusion. While it may have taken a little longer than the norm, much of the credit for this thesis must be ascribed to his dedication to complete and conclusive research. I also wish to thank Dr. Earl Krakower for the many nuclear magnetic resonance spectra he so graciously completed in the course of elucidating the many structures formed and to Dr. -

WO 2014/152434 A2 25 September 2014 (25.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/152434 A2 25 September 2014 (25.09.2014) P O P C T (51) International Patent Classification: (74) Agents: HEBERT, Michael, L. et al; Jones Day, 222 East A61B 17/70 (2006.01) 41st Street, New York, NY 10017-6702 (US). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/US2014/027337 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (22) International Filing Date: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 14 March 2014 (14.03.2014) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, (26) Publication Language: English MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (30) Priority Data: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 61/799,255 15 March 2013 (15.03.2013) US SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, 61/857,174 22 July 2013 (22.07.2013) US TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, 61/876,610 11 September 2013 ( 11.09.2013) us ZW. 61/945,082 26 February 2014 (26.02.2014) us (84) Designated States (unless otherwise indicated, for every 61/945,109 26 February 2014 (26.02.2014) us kind of regional protection available): ARIPO (BW, GH, (71) Applicant: GENOMATICA, INC. -

Hazardous Substances (Chemicals) Transfer Notice 2006
16551655 OF THURSDAY, 22 JUNE 2006 WELLINGTON: WEDNESDAY, 28 JUNE 2006 — ISSUE NO. 72 ENVIRONMENTAL RISK MANAGEMENT AUTHORITY HAZARDOUS SUBSTANCES (CHEMICALS) TRANSFER NOTICE 2006 PURSUANT TO THE HAZARDOUS SUBSTANCES AND NEW ORGANISMS ACT 1996 1656 NEW ZEALAND GAZETTE, No. 72 28 JUNE 2006 Hazardous Substances and New Organisms Act 1996 Hazardous Substances (Chemicals) Transfer Notice 2006 Pursuant to section 160A of the Hazardous Substances and New Organisms Act 1996 (in this notice referred to as the Act), the Environmental Risk Management Authority gives the following notice. Contents 1 Title 2 Commencement 3 Interpretation 4 Deemed assessment and approval 5 Deemed hazard classification 6 Application of controls and changes to controls 7 Other obligations and restrictions 8 Exposure limits Schedule 1 List of substances to be transferred Schedule 2 Changes to controls Schedule 3 New controls Schedule 4 Transitional controls ______________________________ 1 Title This notice is the Hazardous Substances (Chemicals) Transfer Notice 2006. 2 Commencement This notice comes into force on 1 July 2006. 3 Interpretation In this notice, unless the context otherwise requires,— (a) words and phrases have the meanings given to them in the Act and in regulations made under the Act; and (b) the following words and phrases have the following meanings: 28 JUNE 2006 NEW ZEALAND GAZETTE, No. 72 1657 manufacture has the meaning given to it in the Act, and for the avoidance of doubt includes formulation of other hazardous substances pesticide includes but