View PDF Version
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reduction by the Methylreductase System in Methanobacterium Bryantii WILLIAM B
JOURNAL OF BACTERIOLOGY, Jan. 1987, p. 87-92 Vol. 169, No. 1 0021-9193/87/010087-06$02.00/0 Copyright © 1987, American Society for Microbiology Inhibition by Corrins of the ATP-Dependent Activation and CO2 Reduction by the Methylreductase System in Methanobacterium bryantii WILLIAM B. WHITMAN'* AND RALPH S. WOLFE2 Department of Microbiology, University of Georgia, Athens, Georgia 30602,1 and Department of Microbiology, University ofIllinois, Urbana, Illinois 618012 Received 1 August 1986/Accepted 28 September 1986 Corrins inhibited the ATP-dependent activation of the methylreductase system and the methyl coenzyme M-dependent reduction of CO2 in extracts of Methanobacterium bryantii resolved from low-molecular-weight factors. The concentrations of cobinamides and cobamides required for one-half of maximal inhibition of the ATP-depen4ent activation were between 1 and 5 ,M. Cobinamides were more inhibitory at lower concentra- tiops than cobamides. Deoxyadenosylcobalamin was not inhibitory at concentrations up to 25 ,uM. The inhibition of CO2 reduction was competitive with respect to CO2. The concentration of methylcobalamin required for one-half of maximal inhibition was 5 ,M. Other cobamideg inhibited at similar concentrations, but diaquacobinami4e inhibited at lower concentrations. With respect to their affinities and specificities for corrins, inhibition of both the ATP-dependent activation'and CO2 reduction closely resembled the corrin- dependent activation of the methylreductase described in similar extracts (W. B. Whitman and R. S. Wolfe, J. Bacteriol. 164:165-172, 1985). However, whether the multiple effects of corrins are due to action at a single site is unknown. The effect of corrins (cobamides and cobinamides) on in CO2 reduction. -

Identification of Active Methylotroph Populations in an Acidic Forest Soil
Microbiology (2002), 148, 2331–2342 Printed in Great Britain Identification of active methylotroph populations in an acidic forest soil by stable- isotope probing Stefan Radajewski,1 Gordon Webster,2† David S. Reay,3‡ Samantha A. Morris,1 Philip Ineson,4 David B. Nedwell,3 James I. Prosser2 and J. Colin Murrell1 Author for correspondence: J. Colin Murrell. Tel: j44 24 7652 2553. Fax: j44 24 7652 3568. e-mail: cmurrell!bio.warwick.ac.uk 1 Department of Biological Stable-isotope probing (SIP) is a culture-independent technique that enables Sciences, University of the isolation of DNA from micro-organisms that are actively involved in a Warwick, Coventry CV4 7AL, UK specific metabolic process. In this study, SIP was used to characterize the active methylotroph populations in forest soil (pH 35) microcosms that were exposed 2 Department of Molecular 13 13 13 13 and Cell Biology, to CH3OH or CH4. Distinct C-labelled DNA ( C-DNA) fractions were resolved University of Aberdeen, from total community DNA by CsCl density-gradient centrifugation. Analysis of Institute of Medical 16S rDNA sequences amplified from the 13C-DNA revealed that bacteria related Sciences, Foresterhill, Aberdeen AB25 2ZD, UK to the genera Methylocella, Methylocapsa, Methylocystis and Rhodoblastus had assimilated the 13C-labelled substrates, which suggested that moderately 3 Department of Biological Sciences, University of acidophilic methylotroph populations were active in the microcosms. Essex, Wivenhoe Park, Enrichments targeted towards the active proteobacterial CH3OH utilizers were Colchester, Essex CO4 3SQ, successful, although none of these bacteria were isolated into pure culture. A UK parallel analysis of genes encoding the key enzymes methanol dehydrogenase 4 Department of Biology, and particulate methane monooxygenase reflected the 16S rDNA analysis, but University of York, PO Box 373, YO10 5YW, UK unexpectedly revealed sequences related to the ammonia monooxygenase of ammonia-oxidizing bacteria (AOB) from the β-subclass of the Proteobacteria. -

Adaptive Laboratory Evolution Enhances Methanol Tolerance and Conversion in Engineered Corynebacterium Glutamicum
ARTICLE https://doi.org/10.1038/s42003-020-0954-9 OPEN Adaptive laboratory evolution enhances methanol tolerance and conversion in engineered Corynebacterium glutamicum Yu Wang 1, Liwen Fan1,2, Philibert Tuyishime1, Jiao Liu1, Kun Zhang1,3, Ning Gao1,3, Zhihui Zhang1,3, ✉ ✉ 1234567890():,; Xiaomeng Ni1, Jinhui Feng1, Qianqian Yuan1, Hongwu Ma1, Ping Zheng1,2,3 , Jibin Sun1,3 & Yanhe Ma1 Synthetic methylotrophy has recently been intensively studied to achieve methanol-based biomanufacturing of fuels and chemicals. However, attempts to engineer platform micro- organisms to utilize methanol mainly focus on enzyme and pathway engineering. Herein, we enhanced methanol bioconversion of synthetic methylotrophs by improving cellular tolerance to methanol. A previously engineered methanol-dependent Corynebacterium glutamicum is subjected to adaptive laboratory evolution with elevated methanol content. Unexpectedly, the evolved strain not only tolerates higher concentrations of methanol but also shows improved growth and methanol utilization. Transcriptome analysis suggests increased methanol con- centrations rebalance methylotrophic metabolism by down-regulating glycolysis and up- regulating amino acid biosynthesis, oxidative phosphorylation, ribosome biosynthesis, and parts of TCA cycle. Mutations in the O-acetyl-L-homoserine sulfhydrylase Cgl0653 catalyzing formation of L-methionine analog from methanol and methanol-induced membrane-bound transporter Cgl0833 are proven crucial for methanol tolerance. This study demonstrates the importance of -
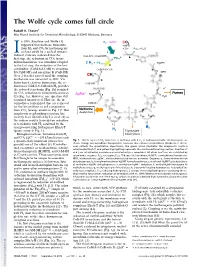
The Wolfe Cycle Comes Full Circle
The Wolfe cycle comes full circle Rudolf K. Thauer1 Max Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany n 1988, Rouvière and Wolfe (1) H - ΔμNa+ 2 CO2 suggested that methane formation + MFR from H and CO by methanogenic + 2H+ *Fd + H O I 2 2 ox 2 archaea could be a cyclical process. j O = Indirect evidence indicated that the CoB-SH + CoM-SH fi *Fd 2- a rst step, the reduction of CO2 to for- red R mylmethanofuran, was somehow coupled + * H MPT 2 H2 Fdox 4 to the last step, the reduction of the het- h erodisulfide (CoM-S-S-CoB) to coenzyme CoM-S-S-CoB b MFR M (CoM-SH) and coenzyme B (CoB-SH). H Over 2 decades passed until the coupling C 4 10 mechanism was unraveled in 2011: Via g flavin-based electron bifurcation, the re- CoB-SH duction of CoM-S-S-CoB with H provides 2 H+ the reduced ferredoxin (Fig. 1h) required c + Purines for CO2 reduction to formylmethanofuran ΔμNa + H MPT 4 f H O (2) (Fig. 1a). However, one question still 2 remained unanswered: How are the in- termediates replenished that are removed CoM-SH for the biosynthesis of cell components H Methionine d from CO2 (orange arrows in Fig. 1)? This Acetyl-CoA e anaplerotic (replenishing) reaction has F420 F420H2 recently been identified by Lie et al. (3) as F420 F420H2 the sodium motive force-driven reduction H i of ferredoxin with H2 catalyzed by the i energy-converting hydrogenase EhaA-T H2 (green arrow in Fig. -

Annotation Guidelines for Experimental Procedures
Annotation Guidelines for Experimental Procedures Developed By Mohammed Alliheedi Robert Mercer Version 1 April 14th, 2018 1- Introduction and background information What is rhetorical move? A rhetorical move can be defined as a text fragment that conveys a distinct communicative goal, in other words, a sentence that implies an author’s specific purpose to readers. What are the types of rhetorical moves? There are several types of rhetorical moves. However, we are interested in 4 rhetorical moves that are common in the method section of a scientific article that follows the Introduction Methods Results and Discussion (IMRaD) structure. 1- Description of a method: It is concerned with a sentence(s) that describes experimental events (e.g., “Beads with bound proteins were washed six times (for 10 min under rotation at 4°C) with pulldown buffer and proteins harvested in SDS-sample buffer, separated by SDS-PAGE, and analyzed by autoradiography.” (Ester & Uetz, 2008)). 2- Appeal to authority: It is concerned with a sentence(s) that discusses the use of standard methods, protocols, and procedures. There are two types of this move: - A reference to a well-established “standard” method (e.g., the use of a method like “PCR” or “electrophoresis”). - A reference to a method that was previously described in the literature (e.g., “Protein was determined using fluorescamine assay [41].” (Larsen, Frandesn and Treiman, 2001)). 3- Source of materials: It is concerned with a sentence(s) that lists the source of biological materials that are used in the experiment (e.g., “All microalgal strains used in this study are available at the Elizabeth Aidar Microalgae Culture Collection, Department of Marine Biology, Federal Fluminense University, Brazil.” (Larsen, Frandesn and Treiman, 2001)). -

Untargetted Metabolomic Exploration of the Mycobacterium Tuberculosis Stress Response to Cinnamon Essential Oil
biomolecules Article Untargetted Metabolomic Exploration of the Mycobacterium tuberculosis Stress Response to Cinnamon Essential Oil Elwira Sieniawska 1,* , Rafał Sawicki 2 , Joanna Golus 2 and Milen I. Georgiev 3,4 1 Chair and Department of Pharmacognosy, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland 2 Chair and Department of Biochemistry and Biotechnology, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland; [email protected] (R.S.); [email protected] (J.G.) 3 Group of Plant Cell Biotechnology and Metabolomics, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, 139 Ruski Blvd., 4000 Plovdiv, Bulgaria; [email protected] 4 Center of Plant Systems Biology and Biotechnology, 4000 Plovdiv, Bulgaria * Correspondence: [email protected] Received: 6 January 2020; Accepted: 24 February 2020; Published: 26 February 2020 Abstract: The antimycobacterial activity of cinnamaldehyde has already been proven for laboratory strains and for clinical isolates. What is more, cinnamaldehyde was shown to threaten the mycobacterial plasma membrane integrity and to activate the stress response system. Following promising applications of metabolomics in drug discovery and development we aimed to explore the mycobacteria response to cinnamaldehyde within cinnamon essential oil treatment by untargeted liquid chromatography–mass spectrometry. The use of predictive metabolite pathway analysis and description of produced lipids enabled the evaluation of the stress symptoms shown by bacteria. This study suggests that bacteria exposed to cinnamaldehyde could reorganize their outer membrane as a physical barrier against stress factors. They probably lowered cell wall permeability and inner membrane fluidity, and possibly redirected carbon flow to store energy in triacylglycerols. Being a reactive compound, cinnamaldehyde may also contribute to disturbances in bacteria redox homeostasis and detoxification mechanisms. -

Towards Kinetic Modeling of Global Metabolic Networks with Incomplete Experimental Input on Kinetic Parameters
Towards Kinetic Modeling of Global Metabolic Networks with Incomplete Experimental Input on Kinetic Parameters Ping Ao 1,2 , Lik Wee Lee 1, Mary E. Lidstrom 3,4 , Lan Yin 5, and Xiaomei Zhu 6 1 Department of Mechanical Engineering , University of Washington , Seattle , WA 98195, USA 2 Department of Physics , University of Washington , Seattle , WA 98195, USA 3 Department of Microbiology , University of Washington , Seattle , WA 98195, USA 4 Department of Chemical Engineering , University of Washington , Seattle , WA 98195, USA 5 School of Physics , Peking University , 100871 Beijing , PR China 6 GeneMath , 5525 27 th Ave . N .E., Seattle , WA 98105, USA Abstract: This is the first report on a systematic method for constructing a large scale kinetic metabolic model and its initial application to the modeling of central metabolism of Methylobacterium extorquens AM1, a methylotrophic and environmental important bacterium. Its central metabolic network includes formaldehyde metabolism, serine cycle, citric acid cycle, pentose phosphate pathway, gluconeogensis, PHB synthesis and acetyl-CoA conversion pathway, respiration and energy metabolism. Through a systematic and consistent procedure of finding a set of parameters in the physiological range we overcome an outstanding difficulty in large scale kinetic modeling: the requirement for a massive number of enzymatic reaction parameters. We are able to construct the kinetic model based on general biological considerations and incomplete experimental kinetic parameters. Our method consists of -

Isolation, Characterization and Substrate-Transport Studies of a New, Unique Methylotroph
RICE UNIVERSITY ISOLATION, CHARACTERIZATION AND SUBSTRATE-TRANSPORT STUDIES OF A NEW, UNIQUE METHYLOTROPH by Thomas Alan Keuer A THESIS SUBMITTED IN PARTIAL FULFULLMENT OF THE REQUIREMENTS FOR THE DEGREE Master of Science APPROVED, THESIS COMMITTEE: E. Terry Papoutsakis, Assistant Professor of Chemical Engineering LarryOf. Mclntire, Professor and Chairman of Chemical Engineering <03^ ' Roger Storck, Professor of Biology Houston, Texas April, 1984 3 1272 00289 0232 ABSTRACT Keuer, Thomas A. M.S. Rice University, April 1984. Isolation, Characterization and Substrate-Transport Studies of a New, Unique Methylotroph. Major Professor: E. T. Papoutsakis. Methylotrophic bacteria which assimilate carbon via the Ribulose Monophosphate Pathway are bioenergetically superior to other methylotrophs. The dehydrogenases which catalyze the oxidation of formaldehyde to formate and formate to CO2 in RMP bacteria produce much of the ATP required for biosynthesis. A strain, designated T15, has been isolated on the basis of high In vitro activities of the above two key enzymes, and has been biochemically characterized. The new strain exhibits high yields (up to 0.63 g cells/g MeOH) and growth rates (up to 0.46 hr“^) in batch culture? however, the yields and growth rates in continuous culture are significantly lower. Study of the transport mechanisms has provided valuable insight into the relationship between substrate uptake and the growth characteristics of T15. Experi¬ ments with radiolabelled substrates have indicated that methanol enters the cells primarily by diffusion? consequently, the bacteria are not able to accumulate methanol internally in order to support efficient Ill continuous growth. Formaldehyde, on the other hand, is accumulated by an active transport system which depends on the A pH component of the membrane proton-motive force. -

Bacterial Metabolism of Methylated Amines and Identification of Novel Methylotrophs in Movile Cave
The ISME Journal (2015) 9, 195–206 & 2015 International Society for Microbial Ecology All rights reserved 1751-7362/15 www.nature.com/ismej ORIGINAL ARTICLE Bacterial metabolism of methylated amines and identification of novel methylotrophs in Movile Cave Daniela Wischer1, Deepak Kumaresan1,4, Antonia Johnston1, Myriam El Khawand1, Jason Stephenson2, Alexandra M Hillebrand-Voiculescu3, Yin Chen2 and J Colin Murrell1 1School of Environmental Sciences, University of East Anglia, Norwich, UK; 2School of Life Sciences, University of Warwick, Coventry, UK and 3Department of Biospeleology and Karst Edaphobiology, Emil Racovit¸a˘ Institute of Speleology, Bucharest, Romania Movile Cave, Romania, is an unusual underground ecosystem that has been sealed off from the outside world for several million years and is sustained by non-phototrophic carbon fixation. Methane and sulfur-oxidising bacteria are the main primary producers, supporting a complex food web that includes bacteria, fungi and cave-adapted invertebrates. A range of methylotrophic bacteria in Movile Cave grow on one-carbon compounds including methylated amines, which are produced via decomposition of organic-rich microbial mats. The role of methylated amines as a carbon and nitrogen source for bacteria in Movile Cave was investigated using a combination of cultivation studies and DNA stable isotope probing (DNA-SIP) using 13C-monomethylamine (MMA). Two newly developed primer sets targeting the gene for gamma-glutamylmethylamide synthetase (gmaS), the first enzyme of the recently-discovered indirect MMA-oxidation pathway, were applied in functional gene probing. SIP experiments revealed that the obligate methylotroph Methylotenera mobilis is one of the dominant MMA utilisers in the cave. DNA-SIP experiments also showed that a new facultative methylotroph isolated in this study, Catellibacterium sp. -

Adverse Effect of the Methanotroph Methylocystis Sp. M6 on the Non-Methylotroph Microbacterium Sp
J. Microbiol. Biotechnol. (2018), 28(10), 1706–1715 https://doi.org/10.4014/jmb.1804.04015 Research Article Review jmb Adverse Effect of the Methanotroph Methylocystis sp. M6 on the Non-Methylotroph Microbacterium sp. NM2 So-Yeon Jeong1, Kyung-Suk Cho2, and Tae Gwan Kim1* 1Department of Microbiology, Pusan National University, Pusan 46241, Republic of Korea 2Department of Environmental Science and Engineering, Ewha Womans University, Seoul 03760, Republic of Korea Received: April 12, 2018 Revised: July 19, 2018 Several non-methylotrophic bacteria have been reported to improve the growth and activity of Accepted: August 23, 2018 methanotrophs; however, their interactions remain to be elucidated. We investigated the First published online interaction between Methylocystis sp. M6 and Microbacterium sp. NM2. A batch co-culture August 24, 2018 experiment showed that NM2 markedly increased the biomass and methane removal of M6. *Corresponding author qPCR analysis revealed that NM2 enhanced both the growth and methane-monooxygenase Phone: +82-515102268; gene expression of M6. A fed-batch experiment showed that co-culture was more efficient in Fax: +82-515141778; -1 -1 E-mail: [email protected] removing methane than M6 alone (28.4 vs. 18.8 µmol·l ·d ), although the biomass levels were similar. A starvation experiment for 21 days showed that M6 population remained stable while NM2 population decreased by 66% in co-culture, but the results were opposite in pure cultures, indicating that M6 may cross-feed growth substrates from NM2. These results indicate that M6 apparently had no negative effect on NM2 when M6 actively proliferated with methane. Interestingly, a batch experiment involving a dialysis membrane indicates that physical proximity between NM2 and M6 is required for such biomass and methane removal enhancement. -

Enzymatic Conversion of CO2 to Methanol
Verónica Catarina Ferreira Amado Licenciada em Bioquímica “One-pot” enzymatic conversion of CO2 to methanol Dissertação para obtenção do Grau de Mestre em Biotecnologia Orientador: Susana Barreiros, Professora Associada com Agregação Universidade Nova de Lisboa Co-orientador: Alexandre Paiva, Investigador Doutorado Universidade Nova de Lisboa Júri: Presidente: Prof. Doutor Carlos Alberto Gomes Salgueiro Arguente: Doutora Ana Sofia Diogo Ferreira Vogal: Prof. Doutora Susana Filipe Barreiros Setembro de 2013 i ii “One-pot” enzymatic conversion of CO2 to methanol Copyright Verónica Catarina Ferreira Amado, FCT/UNL, UNL A Faculdade de Ciências e Tecnologia e a Universidade Nova de Lisboa têm o direito, perpétuo e sem limites geográficos, de arquivar e publicar esta dissertação através de exemplares impressos reproduzidos em papel ou de forma digital, ou por qualquer outro meio conhecido ou que venha a ser inventado, e de a divulgar através de repositórios científicos e de admitir a sua cópia e distribuição com objetivos educativos ou de investigação, não comerciais, desde que seja dado crédito ao autor e editor. iii iv Ao meu irmão Rafael v AGRADECIMENTOS Gostaria de agradecer a todos aqueles que me apoiaram nesta jornada e que, de algum modo contribuíram para a realização desta tese. Quero expressar a mais sincera gratidão aos meus orientadores. Em primeiro lugar à minha orientadora Prof. Doutora Susana Barreiros por todo o interesse e entusiasmo que demonstrou no meu trabalho, por todas as críticas científicas que tanto o enriqueceram, e por estar sempre disponível para discutir o mesmo. Gostaria de agradecer também ao meu co-orientador o Doutor Alexandre Paiva pela paciência, concelhos dados, discussão de ideias e alternativas, pelo espírito crítico sempre incisivo em pontos fulcrais e também pelos momentos de descontracção. -

Enzyme Immobilization for Use in Biofuel Cells and Sensors
(19) & (11) EP 2 343 766 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 13.07.2011 Bulletin 2011/28 H01M 8/16 (2006.01) C12Q 1/00 (2006.01) (21) Application number: 10179649.8 (22) Date of filing: 21.11.2003 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Minteer, Shelly, D. HU IE IT LI LU MC NL PT RO SE SI SK TR Pacific, MO 63069 (US) Designated Extension States: • Akers, Niki, L. AL LT LV MK St Louis, MO 63129 (US) • Moore, Christine, M. (30) Priority: 27.11.2002 US 429829 P St Louis, MO 63125 (US) 10.07.2003 US 486076 P 11.07.2003 US 617452 (74) Representative: Smaggasgale, Gillian Helen W.P. Thompson & Co (62) Document number(s) of the earlier application(s) in 55 Drury Lane accordance with Art. 76 EPC: London WC2B 5SQ (GB) 03812443.4 / 1 565 957 Remarks: (71) Applicant: ST. LOUIS UNIVERSITY This application was filed on 24-09-2010 as a St. Louis, MO 63110-0250 (US) divisional application to the application mentioned under INID code 62. (54) Enzyme immobilization for use in biofuel cells and sensors (57) Disclosed are bioanodes comprising a quater- cleotide. The ion conducting polymer membrane lies jux- nary ammonium treated Nation(R) polymer membrane taposed to a polymethylene green redox polymer mem- and a dehydrogenase incorporated within the treated Na- brane, which serves to electro-oxidize the reduced ade- tion(R) polymer. The dehydrogenase catalyzes the oxi- nine dinucleotide.