X-ADH Is the Sole Alcohol Dehydrogenase Isozyme of Mammalian Brains: Implications and Inferences (Class III Alcohol Dehydrogenase/Tissue-Specific Enzyme) THOMAS B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
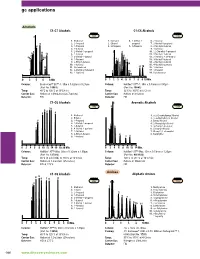
Gc Applications
gc applications Alcohols C1-C7 Alcohols C1-C6 Alcohols 1007 1255 1. Methanol 1. Methanol 4. 2-Methyl-2- 6. 2-Butanol 2. 2-Propanol 2. Ethanol propanol 7. 2-Methyl-1-propanol 3. 1-Propanol 3. 2-Propanol 5. 1-Propanol 8. 2-Methyl-2-butanol 4. 2-Butanol 9. 1-Butanol 5. 2-Methyl-1-propanol 10. 2,2-Dimethyl-1-propanol 6. 1-Butanol 11. 3-Methyl-2-butanol 7. 3-Methyl-1-butanol 12. 2-Pentanol + 3-Pentanol 8. 1-Pentanol 13. 3-Methyl-1-butanol 9. 2-Ethyl-1-butanol 14. 2-Methyl-1-butanol 10. 1-Hexanol 15. 4-Methyl-2-pentanol 11. Cyclohexanol 16. 1-Pentanol 12. 3-Methylcyclohexanol 17. 1-Hexanol 13. 1-Heptanol 18. Cyclohexanol å-IN Column: Econo-Cap™ EC™-1, 30m x 0.32mm x 0.25µm Column: Heliflex® AT™-1, 30m x 0.53mm x 5.00µm (Part No. 19651) (Part No. 16843) Temp: 40°C to 120°C at 10°C/min Temp: 35°C to 100°C at 5°C/min Carrier Gas: Helium at 1.09mL/min (26.7cm/sec) Carrier Gas: Helium at 3mL/min Detector: FID Detector: FID C1-C6 Alcohols Aromatic Alcohols 2596 1249 1. Methanol 1. A,A-Dimethylbenzyl Alcohol 2. Ethanol 2. DL-A-Methylbenzyl Alcohol 3. 1-Propanol 3. Benzyl Alcohol 4. 2-Methyl-1-propanol 4. 2-Phenylethyl Alcohol 5. 1-Butanol 5. 3-Phenyl-1-propanol 6. 4-Methyl-2-pentanol 6. Cinnamyl Alcohol 7. 1-Pentanol 7. Phenyl-1,2-ethanediol 8. 2-Ethyl-1-butanol 8. -

Isozymes: Methods and Applications*
Chapter 9 Isozymes: Methods and Applications* J. A. Micales and M. R. Bonde TABLE OF CONTENTS I. Introduction . II. Principles A. Multiple Alleles at Single Locus B. Single or Multiple Alleles at Multiple Loci C. Secondary Isozymes III. Methodology A. Sample selection and Preparation B. Electrophoretic Techniques C. Staining D. Genetic Interpretation IV. Applications of Isozyme Analysis A. Taxonomy B. Identification of Unknown Organisms C. Genetics D. Epidemiology E. Pathogenicity and Virulence V. Advantages and Disadvantages VI. Conclusions References Recommended Reading Keywords – Isozymes, plant pathology, genetics, taxonomy I. INTRODUCTION Isozyme analysis is a powerful biochemical technique with numerous applications in plant pathology. It has long been used by geneticists to study the population genetics of fish, mammals, insects, nematodes, and higher plants. Mycologists and plant pathologists more recently adopted the procedure, and it is now being used routinely to settle taxonomic disputes, identify “unknown” cultures, “fingerprint” patentable fungal lines and plant cultivars, analyze genetic variability, trace pathogen spread, follow the segregation of genetic loci, and determine ploidy levels of fungi and other plant pathogens. These topics have been recently reviewed.1,2 The large number of publications in this field each year indicates the widespread interest in isozyme analysis. In this paper, we discuss some major applications of isozyme analysis in basic and applied plant pathology. The technique is particularly useful with fungi; the greatest advances have been mostly with fungal pathogens. Isozyme banding patterns obtained from fungi are usually relatively uncomplicated and easy to interpret. Isozyme analysis can be readily performed in most laboratories with relatively little expense. With the development of computer programs that enable large numbers of comparisons at the gene level, much information cart be obtained about the population genetics and life cycle of the organism. -

A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing A-Cyano Or A-Ester Functional Group from Baylis-Hillman Adducts
Notes Bull. Korean Chem. Soc. 2004, Vol. 25, No. 3 413 A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing a-Cyano or a-Ester Functional Group from Baylis-Hillman Adducts Ka Young Lee, Saravanan GowriSankar, and Jae Nyoung Kim Department of Chemistry and Institute of Basic Science, Chonnam National University, Gwangju 500-757, Korea Received December 18, 2003 Key Words : Cinnamyl alcohols, Baylis-Hillman adducts, Sulfuric acid, Acetic anhydride Cinnamyl alcohols bearing ester, acid, or nitrile functional Hillman alcohol into the primary acetate or eventually to group at the 2-position are very useful synthons for the primary alcohol (Scheme 1 and Table 1). synthesis of various biologically important compounds.1 The reaction of Baylis-Hillman adducts with acetic Synthesis of cinnamyl alcohol derivatives from the Baylis- anhydride (1.5 equiv.) in the presence of sulfuric acid (10 Hillman adducts has been reported by us and other groups.1-4 mol%) gave the corresponding rearranged cinnamyl acetates The conversion can be carried out directly by using 20% quantitatively in dichloromethane at room temperature aqueous sulfuric acid (for the Baylis-Hillman adducts within 30 min (Actually, the cinnamyl acetate derivatives derived from acrylonitrile)2 or trifluoroacetic acid (for the could be isolated in 92-95% yields.). In order to obtain the Baylis-Hillman adducts derived from acrylates).3 Recently, corresponding cinnamyl alcohols in a one-pot we tried some Basavaiah and coworkers have reported the two-step, one- reaction conditions for the next partial hydrolysis. Addition pot conversion method: TMSOTf-assisted conversion of of water to the reaction mixture (two-phase system) afforded Baylis-Hillman alcohol into the corresponding primary the hydrolyzed cinnamyl alcohols in trace amounts. -

Human Glucokinase Gene
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 7698-7702, August 1992 Genetics Human glucokinase gene: Isolation, characterization, and identification of two missense mutations linked to early-onset non-insulin-dependent (type 2) diabetes mellitus (glucose/metabolism/phosphorylation/structure4unctlon/chromosome 7) M. STOFFEL*, PH. FROGUELt, J. TAKEDA*, H. ZOUALItt, N. VIONNET*, S. NISHI*§, I. T. WEBER¶, R. W. HARRISON¶, S. J. PILKISII, S. LESAGEtt, M. VAXILLAIREtt, G. VELHOtt, F. SUNtt, F. lIRSt, PH. PASSAt, D. COHENt, AND G. I. BELL*"** *Howard Hughes Medical Institute, and Departments of Biochemistry and Molecular Biology, and of Medicine, The University of Chicago, 5841 South Maryland Avenue, MC1028, Chicago, IL 60637; §Second Division of Internal Medicine, Hamamatsu University School of Medicine, Hamamatsu, Shizuoka 431-32, Japan; IDepartment of Pharmacology, Jefferson Cancer Institute, Thomas Jefferson University, Philadelphia, PA 19107; IlDepartment of Physiology and Biophysics, State University of New York, Stony Brook, NY 11794; tCentre d'Etude du Polymorphisme Humain, 27 rue Juliette Dodu, and Service d'Endocrinologie, H6pital Saint-Louis, 75010 Paris, France; and tG6ndthon, 1 rue de l'Internationale, 91000 Evry, France Communicated by Jean Dausset, May 28, 1992 ABSTRACT DNA polymorphisms in the glucokinase gene by maintaining a gradient for glucose transport into these cells have recently been shown to be tightly linked to early-onset thereby regulating hepatic glucose disposal. In (3 cells, glu- non-insulin-dependent diabetes mellitus in "80% of French cokinase is believed to be part of the glucose-sensing mech- families with this form of diabetes. We previously identified a anism and to be involved in the regulation ofinsulin secretion. -

Practical Isozyme Genetics by N. Pasteur, G. Pasteur, F. Bonhomme
Book reviews 244 throughout its development, based on a combination and sexual selection, which also reports many interes- of cell lineage studies and reconstructions of electron ting discoveries. The primary sex-determining signal is micrographs of serial sections, has made possible a the X/A ratio, which appears to set the states of a genetic analysis of the control of cell lineage by studies small number of sex-determining genes, which then of a large number of mutants which distort particular direct development along a particular sexual pathway. cell lineages. Many types of such mutants remain to be Of the seven major genes so far identified, the three tra identified by new types of selection procedure, but this genes appear to be required in the hermaphrodite but work is at the stage where molecular analysis of not in the male, the her-1 gene is required in the male lineage mutants is possible - obviously a very im- only, and the three fern genes are required for male portant area for experimental embryology. somatic development and also for spermatogenesis in Muscle anatomy, assembly and function have been both males and hermaphrodites. Dosage compen- studied mainly on the body-wall musculature re- sation is thought to be achieved by increasing sponsible for movement of the animal forwards and transcription from the single X chromosome of the backwards. The major muscle components are similar XO male or decreasing transcription from the two X to those of other animals: muscle extracts contain chromosomes of the XX hermaphrodite. Thus there myosin with both heavy- and light-chain subunits, are similarities between the sex determination and actin, paramyosin, tropomyosin and troponin-like dosage compensation systems of C. -

Food and Drug Administration, HHS § 172.515
Food and Drug Administration, HHS § 172.515 Common name Scientific name Limitations Tolu ................................................................ Myroxylon balsamum (L.) Harms. Turpentine ...................................................... Pinus palustris Mill. and other Pinus spp. which yield terpene oils exclusively. Valerian rhizome and roots ............................ Valeriana officinalis L. Veronica ......................................................... Veronica officinalis L .................................................. Do. Vervain, European ......................................... Verbena officinalis L ................................................... Do. Vetiver ............................................................ Vetiveria zizanioides Stapf ......................................... Do. Violet, Swiss ................................................... Viola calcarata L. Walnut husks (hulls), leaves, and green nuts Juglans nigra L. or J. regia L. Woodruff, sweet ............................................. Asperula odorata L ..................................................... In alcoholic beverages only Yarrow ............................................................ Achillea millefolium L .................................................. In beverages only; fin- ished beverage thujone free1 Yerba santa .................................................... Eriodictyon californicum (Hook, et Arn.) Torr. Yucca, Joshua-tree ........................................ Yucca brevifolia Engelm. Yucca, -

Nomination Background: Methyl Trans
' 7/?1 Methyl styryl ketone 122-57-6 NTP NOMINATION HISTORY AND REVIEW A. Nomination History 1. Nomination Source: NCI 2. Recommendations: -Comparative toxicity studies with methyl vinyl ketone -Metabolism -Carcinogenicity 3. Rationale/Remarks: -Potential for widespread human exposure -Nominated as a result of a class study of a,~-unsaturated ketones -Flavoring and fragrance additive -Natural product -Environmental pollutant -Mutagenic in Salmonella 4. Priority: -High for toxicity studies -Moderate for carcinogenicity s. Date of Nomination: July 1994 B. Interagency Committee for Chemical Evaluation and Coordination Review 1. Date of Review: 2. Recommendations: 3. Priority: 4. NTP Chemical Selection Principles: 5. Rationale/Remarks: 122-57-6 Methyl styryl ketone SUMMARY OF DATA FOR CHEMICAL SELECTION CHEMICAL IDENTIFICATION CAS Registry Number: 122-57-6 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl- (SCI, 9CI) Svnonvms and Trade Names: Acetocinnamone; benzalacetone; benzylideneacetone; methyl 2 phenylvinyl ketone; methyl styryl ketone; methyl ~-styryl ketone; 4 phenylbutenone; 4-phenyl-3-butene-2-one; 2-phenylvinyl methyl ketone; styryl methyl ketone; MSK FEMA Number: 2881 Related isomers CAS Registry Number: 937-53-1 Chemica] Abstracts Name: 3-Buten-2-one, 4-phenyl-, (Z)- (SCI, 9CI) Synonvms and Trade Names: cis -4-Phenyl-3-buten-2-one; cis -benzalacetone; cis benzylideneacetone; cis-methyl styryl ketone CAS Registry Number: 1896-62-4 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl-, (E)- (SCI, 9CI) Synonyms and Trade Names: trans-4-Phenyl-3-buten-2-one; trans-benzalacetone; trans benzylideneacetone; trans-methyl styryl ketone; TPBO Structure. Molecular Formula. and Molecular WeiKbt 0 II H•CHCCH3 Mol. wt.: 146.19 Chemical and Phvsical Properties (from Aldrich Chemical Co., 1993, 1994; Budavari, 1989) Description: White to yellow solid with a sweet, pungent, creamy, floral odor Prepared for NCI by Technical Resources, Inc. -

Immunoelectron Microscopic Localization of 3Β-Hydroxysteroid Dehydrogenase and Type 5 17Β-Hydroxysteroid Dehydrogenase In
11 Immunoelectron microscopic localization of 3-hydroxysteroid dehydrogenase and type 5 17-hydroxysteroid dehydrogenase in the human prostate and mammary gland G Pelletier, V Luu-The, M El-Alfy, S Li and F Labrie Oncology and Molecular Endocrinology Research Center, Laval University Hospital (CHUL), Quebec, G1V 4G2, Canada (Requests for offprints should be addressed to G Pelletier, Oncology and Molecular Endocrinology Research Center, Laval University Hospital (CHUL), 2705, Laurier Boulevard, Quebec, Quebec, G1V 4G2, Canada; Email: [email protected]) ABSTRACT The subcellular distribution of steroidogenic organelle could be observed, although some concen- enzymes has so far been studied mostly in classical tration of gold particles was occasionally found over endocrine glands and in the placenta. In the bundles of microfilaments. In mammary gland peripheral intracrine organs which synthesize sex sections immunolabelled for 3-HSD or type 5 steroids there is no indication about the organelles 17-HSD localization, labelling was observed in the which contain the enzymes involved in steroid cytoplasm of the secretory epithelial cells in both biosynthesis. We have thus investigated the sub- the acini and terminal ducts. Immunolabelling was cellular localization of two enzymes involved in also found in the endothelial cells as well as in the production of sex steroids, namely 3- fibroblasts in stroma and blood vessels. The gold hydroxysteroid dehydrogenase (3-HSD) and type particles were not detected over any organelles, 517-hydroxysteroid dehydrogenase (17-HSD). except with the occasional accumulation of gold Using specific antibodies to these enzymes, we particles over microfilaments. The present data on conducted immunoelectron microscopic studies in the localization of two steroidogenic enzymes two peripheral tissues, namely the human prostate leading to the synthesis of testosterone indicate that and mammary gland. -

Isozyme Studies on the Origin and Evolution of Puccinia Graminis F
Aust. J. Bioi. Sci., 1982,35, 231-8 Isozyme Studies on the Origin and Evolution of Puccinia graminis f. sp. tritici in Australia J. J. Burdon/ D. R. Marshall,A.c N. H. Lui~ and D. J. S. GoW» A Division of Plant Industry, CSIRO, P.O. Box 1600, Canberra City, A.C.T. 2601. B Plant Breeding Institute, University of Sydney, P.O. Box 180, Castle Hill, N.S.W. 2154. C Present address: I. A Watson Wheat Research Centre, University of Sydney, P.O. Box 219, Narrabri, N.S.W. 2390. Abstract Isozyme phenotypes for eight enzyme systems were used to assess the origins and evolution of P. graminis f. sp. tritici (the wheat stem rust pathogen) in Australia. The results obtained by this approach agreed with pathways postulated on the basis of virulence studies, confirming the sugges tion that most of the major changes in the wheat stem rust pathogen flora of Australia have resulted from overseas introductions. Moreover, they suggest that, although the more recent of these were from Africa, the first major change detected occurred as a result of an introduction from elsewhere. Introduction Australia offers unique opportunities for studies in the variation and evolution of the wheat stem rust pathogen, Pucdnia graminis Pers. f. sp. tritid Eriks. & Henn.. Barberry bushes (Berberis vulgaris L.), on which the sexual stage occurs, are extremely rare and the region is isolated from other wheat-growing regions of the world. On the other hand, the occasional arrival of new rust types provides variability additional to that occurring through mutation. -

Neurosteroid Metabolism in the Human Brain
European Journal of Endocrinology (2001) 145 669±679 ISSN 0804-4643 REVIEW Neurosteroid metabolism in the human brain Birgit Stoffel-Wagner Department of Clinical Biochemistry, University of Bonn, 53127 Bonn, Germany (Correspondence should be addressed to Birgit Stoffel-Wagner, Institut fuÈr Klinische Biochemie, Universitaet Bonn, Sigmund-Freud-Strasse 25, D-53127 Bonn, Germany; Email: [email protected]) Abstract This review summarizes the current knowledge of the biosynthesis of neurosteroids in the human brain, the enzymes mediating these reactions, their localization and the putative effects of neurosteroids. Molecular biological and biochemical studies have now ®rmly established the presence of the steroidogenic enzymes cytochrome P450 cholesterol side-chain cleavage (P450SCC), aromatase, 5a-reductase, 3a-hydroxysteroid dehydrogenase and 17b-hydroxysteroid dehydrogenase in human brain. The functions attributed to speci®c neurosteroids include modulation of g-aminobutyric acid A (GABAA), N-methyl-d-aspartate (NMDA), nicotinic, muscarinic, serotonin (5-HT3), kainate, glycine and sigma receptors, neuroprotection and induction of neurite outgrowth, dendritic spines and synaptogenesis. The ®rst clinical investigations in humans produced evidence for an involvement of neuroactive steroids in conditions such as fatigue during pregnancy, premenstrual syndrome, post partum depression, catamenial epilepsy, depressive disorders and dementia disorders. Better knowledge of the biochemical pathways of neurosteroidogenesis and -

Functional Expression of a Novel Methanol-Stable Esterase From
Cai et al. BMC Biotechnology (2020) 20:36 https://doi.org/10.1186/s12896-020-00622-1 RESEARCH ARTICLE Open Access Functional expression of a novel methanol- stable esterase from Geobacillus subterraneus DSM13552 for biocatalytic synthesis of cinnamyl acetate in a solvent- free system Xianghai Cai1†, Lin Lin2,3†, Yaling Shen1, Wei Wei1* and Dong-zhi Wei1 Abstract Background: Esterases are widely distributed in nature and have important applications in medical, industrial and physiological. Recently, the increased demand for flavor esters has prompted the search of catalysts like lipases and esterases. Esterases from thermophiles also show thermal stability at elevated temperatures and have become enzymes of special interest in biotechnological applications. Although most of esterases catalyzed reactions are carried out in toxic and inflammable organic solvents, the solvent-free system owning many advantages such as low cost and easy downstream processing. Results: The gene estGSU753 from Geobacillus subterraneus DSM13552 was cloned, sequenced and overexpressed into Escherichia coli BL21 (DE3). The novel gene has an open reading frame of 753 bp and encodes 250-amino-acid esterase (EstGSU753). The sequence analysis showed that the protein contains a catalytic triad formed by Ser97, Asp196 and His226, and the Ser of the active site is located in the conserved motif Gly95-X-Ser97-X-Gly99 included in most esterases and lipases. The protein catalyzed the hydrolysis of pNP-esters of different acyl chain lengths, and the enzyme specific activity was 70 U/mg with the optimum substrate pNP-caprylate. The optimum pH and temperature of the recombinant enzyme were 8.0 and 60 °C respectively. -

Rearrangement of Allylic Alcohols Herbert Barbehenn
Rochester Institute of Technology RIT Scholar Works Theses Thesis/Dissertation Collections 1-1-1971 Rearrangement of allylic alcohols Herbert Barbehenn Follow this and additional works at: http://scholarworks.rit.edu/theses Recommended Citation Barbehenn, Herbert, "Rearrangement of allylic alcohols" (1971). Thesis. Rochester Institute of Technology. Accessed from This Thesis is brought to you for free and open access by the Thesis/Dissertation Collections at RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. REARRANGEMENT OF ALLYLIC ALCOHOLS HERBERT S. BARBEHENN JANUARY, 1971 THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE APPROVED: Dr. Jerry Adduci Project Adviser Department Head Library Rochester Institute of Technology Rochester, New York To Rath, my wife - - - for the many lonely nights, for the many unfinished chores and for being herself. Acknowledgements Grateful appreciation is tendered to the many faculty members with whom it has been my pleasure to be associated with during the past eleven years at Rochester Institute of Technology. Special thanks are expressed to Dr. Jerry Adduci for his guidance and patience in seeing this endeavor to its conclusion. While it may have taken a little longer than the norm, much of the credit for this thesis must be ascribed to his dedication to complete and conclusive research. I also wish to thank Dr. Earl Krakower for the many nuclear magnetic resonance spectra he so graciously completed in the course of elucidating the many structures formed and to Dr.