Principles of Geochemical Prospecting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geobotanical Prospecting Around the Navan Zn-Pb Deposit, Ireland: Developing Geochemical Vectors for Mineral Exploration
Goldschmidt2017 Abstract Geobotanical Prospecting around the Navan Zn-Pb Deposit, Ireland: Developing geochemical vectors for mineral exploration DANIJELA MAVRIC1,2*, JOHN H. ASHTON1, SEÁN H. MCCLENAGHAN2 & BALZ S. KAMBER2 1Boliden Tara Mines Limited, Navan, Co Meath, Ireland (*correspondence: [email protected]) 2Department of Geology, ScHool of Natural Sciences, Trinity College Dublin, Dublin, Ireland Recent studies Have shown great potential for Using plants in mineral exploration, for example grass root geocHemistry, wHich led to a discovery of a new AUstralian metallogenic province [1]. In this researcH, the trace-element geocHemistry of native and common plant species in Ireland sucH as ash, beecH, blackberry, Hawthorn, oak, poplar and sycamore was systematically determined above the Navan Zn-Pb deposit (Ireland), cUrrently mined by Boliden Tara Mines Limited. THe study area represents a sub-oUtcropping carbonate-Hosted base˗metal ore body characterised by a large shallow soil Zn- Pb anomaly. The new results show that Zn contents in leaf materials across all species vary from 18 ppm to 700 ppm (dry weigHt). THe HigHest concentrations of Zn are observed in poplar trees growing above subcropping mineralization. Levels of Pb and Cd are anomaloUsly HigH in all investigated tree species grown in soil witH metal anomalies, compared to the backgroUnd values from control sites. In this study, focUs was also placed on Hawthorn and oak as a representative plant species Used for spatial and temporal vectoring. Using trace- element analysis, anomalies can be detected, bUt Uncertainties remain concerning their soUrces. In order to better Understand anomaly soUrce several models of Cu and Zn isotope data will illustrate the typical ranges of metal stable isotopes one migHt expect in diverse geological, and botanical setting. -

M.Sc. Plant Science (Applicable to Students Admitted in 2009 Onwards)
M.Sc. Plant Science (Applicable to students admitted in 2009 onwards) (w.e.f. examination of 2009 onwards) M.Sc. I (Previous) Semester I Paper I - Microbiology (Bacteriology, Virology) and Microbial 100 Marks Biotechnology. Paper II - Mycology & Plant Pathology (Fungal Diseases) 100 Marks Paper III - Algology & Lichenology 100 Marks Paper IV - Bryology 100 Marks Practical - Based on Papers I to IV including 200 Marks Class and Field Work (Local excursion) 600 Marks Semester II Paper V - Pteridophytes 100 Marks Paper VI - Gymnosperms and Palaeobotany 100 Marks Paper VII - Angiosperms: Taxonomy and Economic Botany 100 Marks Paper VIII - Angiosperms: Histology, Anatomy, Embryology 100 Marks Practical - Based on Papers V to VIII 200 Marks - Class and Field Work (Local excursion) 600 Marks Students have to take all the eight papers. M.Sc. II (Final) Semester III Paper I - Cytology, Genetics and Cytogenetics 100 Marks Paper II - Plant Breeding and Biostatistics 100 Marks Paper III - Ecology, Environment and Soil Science 100 Marks Paper IV - Modern experimental techniques and computer application 100 Marks Practical - Based on Papers I to IV including 200 Marks - Class and field work/Laboratory visit 600 Marks Semester IV Paper V - Plant Physiology 100 Marks Paper VI - Cell Biology & Plant Biochemistry 100 Marks Paper VII - Biotechnology and Human welfare 100 Marks Paper VIII Elective - Project work (Review based on all Papers from 100 Marks Semester I to IV) Practical - Based on Papers V and VII 200 Marks (Incl. Biotech Lab visits & class work) 600 Marks Students have to take all the seven papers (a) The theory papers would have internal evaluation by the teaching/guest faculty as decided by the Coordinator in accordance with the nature and requirement of the subject and shall be notified to the students in the beginning of the semester. -

Geo Botanical Studies on Obulapuram Iron Mine
GEO BOTANICAL STUDIES ON OBULAPURAM IRON MINE R. M. DHANARAJU A. LAKSHMAIAH , Retired Principal, Reader in Botany, S.K.P. Govt. Degree College, Govt. Degree College, Guntakal. (AP) INDIA. Pattikonda. (AP) INDIA. R.M. VENUGOPAL K. VEERANJANEYULU Retired Principal, Rtd. Prof., Govt. Junior College, S.K. University, Pamidi. (AP) INDIA. Anantapuram (AP) INDIA. In this study Vegetation of Iron mine showed very sparce and comprises theory scrubs and very few tree species, with Semi-evergreen nature. Ninty four species belonging to 80 genera of 30 angiosperm families were recorded on Iron mine. Eight species showed the highest percentage of presence values on Iron mine Schouwia purpurea possessed the highest percentage of presence, density and higher abundance and higher levels on iron accumulation. As Schouja purpurea is the costant species, it may be considered as a local indicator plant for Iron. Key words : Iron mine, constant species, local indicator plant. INTRODUCTION Plants growing on mines and mine relics have been found to accumulate and tolerate unusual concentration on metals. These metal to learnt species as indicator plants have attracted the attention for prospecting the mineral deposits. Individual species or vegetation types are known to act as indicators of are deposits in geobotanical prospecting. Several investigators have recognized different plant associations on varying geologic substrates. Koch(1932) studied in detail the communities growing on zinc and copper contaminated soils. Similarly many geobotanists have reported on the exclusive occurrence of certain plant species and their associations on metal rich soils ( Aery 1977; cole 1965; Nesvetayalova 1961; Tiagi & Singh 1973; Venkatesh 1964: Veeranjaneyulu & Dhanaraju GEO BOTANICAL STUDIES ON OBULAPURAM IRON MINE 1P a g e 1990) Ernst (1966) has successfully classified plant communities on soils containing heavy metals according to Braun – Blanquet (1932) approach. -

(An Autonomous College) BOTANY SYLLABUS
GOVERNMENT COLLEGE FOR WOMEN, PARADE GROUND, JAMMU (An Autonomous College) BOTANY SYLLABUS B.Sc. SEMESTER I-IV UNDER CHOICE BASED CREDIT SYSTEM (CBCS) AND B.Sc. SEMESTER V-VI ----------------------------------------------------------------------------------------------------- GOVERNMENT COLLEGE FOR WOMEN, PARADE GROUND, JAMMU BOTANY (Semester-I) (For examinations to be held in the years 2016, 2017, 2018) Title: Diversity of Microbes & Cryptogams (Theory) Duration of Exam: 3hrs Maximum Marks: 100 Credits: 04 External Examination: 80 Marks Internal Assessment: 20 Marks Objectives: The course is designed to familiarize the students with microbes and cryptogams. These plant groups are of great human use in agriculture, horticulture, medical and biotechnology based industries. Therefore, students need to know about their structural diversity, biology and utilization. Unit-I Microbes and Microbiology 1.1 General account of plant viruses (TMV), transmission and control; concept of viroids and prions. 1.2 Bacteria-Ultrastructure, nutrition and reproduction, general account of Mycoplasma and Cyanobacteria. 1.3 Genetic recombination in bacteria (transformation, transduction and conjugation). 1.4 Economic importance of bacteria and plant viruses. Unit-II Algae 2.1 General characteristics and classification of algae (Parker, 1982) up to class level. 2.2 Important features of Chlorophyceae and Xanthophyceae; life histories of Volvox, Oedogonium, Chara and Vaucheria. 2.3 Important features of Phaeophyceae and Rhodophyceae; Life histories of Ectocarpus and Polysiphonia. 2.4 Economic importance of algae (as food and feed; algal blooms and toxins). Unit-III Fungi 3.1 General characteristics and classification of fungi (Ainsworth 1971), Economic importance of fungi, General account of Lichens. 3.2 Important features of Mastigomycotina; Life histories of Pythium and Allomyces. -

Copper Deposits in Sedimentary and Volcanogenic Rocks
Copper Deposits in Sedimentary and Volcanogenic Rocks GEOLOGICAL SURVEY PROFESSIONAL PAPER 907-C COVER PHOTOGRAPHS 1 . Asbestos ore 8. Aluminum ore, bauxite, Georgia 1 2 3 4 2. Lead ore. Balmat mine, N . Y. 9. Native copper ore, Keweenawan 5 6 3. Chromite-chromium ore, Washington Peninsula, Mich. 4. Zinc ore, Friedensville, Pa. 10. Porphyry molybdenum ore, Colorado 7 8 5. Banded iron-formation, Palmer, 11. Zinc ore, Edwards, N.Y. Mich. 12. Manganese nodules, ocean floor 9 10 6. Ribbon asbestos ore, Quebec, Canada 13. Botryoidal fluorite ore, 11 12 13 14 7. Manganese ore, banded Poncha Springs, Colo. rhodochrosite 14. Tungsten ore, North Carolina Copper Deposits in Sedimentary and Volcanogenic Rocks By ELIZABETH B. TOURTELOT and JAMES D. VINE GEOLOGY AND RESOURCES OF COPPER DEPOSITS GEOLOGICAL SURVEY PROFESSIONAL PAPER 907-C A geologic appraisal of low-temperature copper deposits formed by syngenetic, diagenetic, and epigenetic processes UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1976 UNITED STATES DEPARTMENT OF THE INTERIOR THOMAS S. KLEPPE, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director First printing 1976 Second printing 1976 Library of Congress Cataloging in Publication Data Tourtelot, Elizabeth B. Copper deposits in sedimentary and volcanogenic rocks. (Geology and resources of copper) (Geological Survey Professional Paper 907-C) Bibliography: p. Supt. of Docs. no.: I 19.16:907-C 1. Copper ores. 2. Rocks, Sedimentary. 3. Rocks, Igneous. I. Vine, James David, 1921- joint author. II. Title. III. Series. IV. Series: United States Geological Survey Professional Paper 907-C. TN440.T68 553'.43 76-608039 For sale by the Superintendent of Documents, U.S. -

Wall Rock Alteration in the Kanoko Vein Swarm, Hosokura Mine, Miyagi Prefecture, North-East Japan
Title Wall Rock Alteration in the Kanoko Vein Swarm, Hosokura Mine, Miyagi Prefecture, North-east Japan Author(s) Narita, Eikichi Citation Journal of the Faculty of Science, Hokkaido University. Series 4, Geology and mineralogy, 11(1), 59-75 Issue Date 1961-03 Doc URL http://hdl.handle.net/2115/35924 Type bulletin (article) File Information 11(1)_59-76.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP WALL ROCK ALTERATION IN THE KANOKO VEIN SWARM, HOSOKURA MINE, MIYAGI PREFECTURE, NORTH-EAST JAPAN By Eikichi NARITA Contributions from the Department of Geology and Mineralogy, Faculty of Seienee, Hol<kaido University, No. 828 lntroduction Hosokura mine, enumerated as exploiting one of the big four lead-zinc' deposits in Japan, is loeated in Uguisuzawa IM{achi, Miyagi Prefecture,, northeastern part of Honshu island. Its mining field is composed ex-・ clusively of huge Neogene Tertiary volcanie materials corresponding to・ a part of the long continued belt extending along the whole inner zone of the Japanese islands arc. "Green tuff region" is the name proposed for that prominent Neogene Tertiary effusive terrain, which has recently' become an attractive field for the Japanese geologists as having resulted from epoch-making events in the history of the development of the Japa- nese islands arc. Various metallif'erous deposits are also associated with. those volcanic activities forming a peculiar ore province that has an impoytant- role in respeet to the Japanese mining industry. Epithermal types of deposits, such as chlorite copper veins, lead-zinc' veins and so-called black ore etc. are the characteristic representatives・ of the province. -

Geological Time Scale Lecture Notes
Geological Time Scale Lecture Notes dewansEddy remains validly. ill-judged: Lefty is unhelpable: she leaf her she reinsurers phrases mediates inattentively too artfully?and postmarks Ruthenic her and annual. closed-door Fabio still crenellate his Seventh grade Lesson Geologic Time Mini Project. If i miss a lecture and primitive to copy a classmate's notes find a photocopying. Geologic Time Scale Age of free Earth subdivided into named and dated intervals. The Quaternary is even most recent geological period for time in trek's history spanning the unique two million. Geologic Time and Earth Science Lumen Learning. Explaining Events Study arrangement 3 in Figure B Note that. Do not get notified when each lecture notes to be taken in fact depends on plate boundaries in lecture notes with it was convinced from? Coloured minerals introduction to mining geology lecture notes ppt Mining. The geologic record indicates several ice surges interspersed with periods of. Lecture Notes Geologic Eras Geologic Timescale The geologic timetable is divided into 4 major eras The oldest era is called the Pre-Cambrian Era. 4 Mb Over long periods of debate many rocks change shape and ease as salary are. A Geologic Time Scale Measures the Evolution of Life system Review NotesHighlights Image Attributions ShowHide Details. Lecture notes lecture 26 Geological time scale StuDocu. You are encouraged to work together and review notes from lectures to flex on. These lecture notes you slip and indirect evidence of rocks, and phases and geological time scale lecture notes made by which help you? Index fossil any homicide or plant preserved in the department record write the bond that is characteristic of behavior particular complain of geologic time sensitive environment but useful index. -

Mineralogical Studies on Japanese Sphalerite
Title Mineralogical Studies on Japanese Sphalerite Author(s) Togari, Kenji Citation Journal of the Faculty of Science, Hokkaido University. Series 4, Geology and mineralogy, 10(4), 703-734 Issue Date 1961-03 Doc URL http://hdl.handle.net/2115/35922 Type bulletin (article) File Information 10(4)_703-734.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP MINERALOGICAL STUDIES ON JAPANESE SPHALERXTE By Kenji TOGARI Contributions from the Department of Geology and Mineralogy, Faeulty of Science, Hol<kaido Unive' rsity. No.・ s26 CONTENTS I. Introductiori ........,..,......,.......,..,......,.,...........,,703 II. Oecurrences .....,........,...,.,............,',.,.....,..,,.,...705 III. Morphology of sphalerite .....,..,..,....,...,.,...,...,..,,.....708 III-1 Forms and localities ..................,,...,.,.,,,.,,.....708 III-2 H-, F-, and P-value.......,,...,,.,....,.........,...,..,708 III-3 Twinning ..,.......,...,...............,.,.......,....,..714 IV. Chemical composition of sphalerite..,....,.....,.......,.,........714 IV-1 Chemieal analyses......,.,..........,....,...,.....,.....・・715 IV-2 Sulphur cornponent............,...,..,,......,..,...,....,.717 IV-3 Minor elements.................,.....,.,.,.,..,,...・-・・・・・717 V. Problem on structure of sphalerite......,..,v.,.,....,...........720 V-1 Variation of lattice eonstant...,....,.,...,.......,...,.....721 V-2 PolymoT'phism and polytype ..,...,......,......,..........,724 VI. Colour of sphalerite,...........................,...........,.,...725 -

Mining, Geology, and Geological History of Garnet at the Barton Garnet Mine, Gore Mountain, New York William Kelly
6: RARE EARTH ELEMENT AND YTTRIUM MINERAL OCCURENCES IN THE ADIRONDACK MOUNTAINS 7 MINING, GEOLOGY, AND GEOLOGICAL HISTORY OF GARNET AT THE BARTON GARNET MINE, GORE MOUNTAIN, NEW YORK WILLIAM KELLY New York State Geologist, Emeritus, Division of Research and Collections, New York State Museum, Albany, NY 12230, [email protected] KEYWORDS: Garnet, Mining, Gore Mountain, Metamorphism, Lyon Mountain Granite ABSTRACT Garnet megacrysts commonly 30 centimters (cm) ranging up to 1 meter (m) in diameter occur at the summit of Gore Mountain, Adirondacks, NY and were mined there for abrasives for more than a century. The mine, owned by Barton Mines Co., LLC, is roughly 2 km x 150 m and is located in a hornblende-rich garnet amphibolite at the southern boundary of a metamorphosed olivine gabbro body that is in fault contact with charnockite. Barton supplies garnet, a chemically homogeneous pyrope-almandine, to the waterjet cutting, lapping, and abrasive coatings industries. The garnet megacrysts are reliably dated at 1049 ± 5 Ma. The growth of the garnet megacrysts was facilitated by an influx of hydrothermal fluid emanating from the ore body’s southern boundary fault. The fluids were most probably associated with the intrusion of the Lyon Mountain Granite (1049.9 ± 10 Ma) and/or associated pegmatitic rocks late in the tectonic history of the Adirondacks. INTRODUCTION The Adirondack Mountains in upstate New York are a small outlier of a larger body of rocks of similar age and geologic history that is located to the north in Canada. The Adirondack region can be loosely divided into amphibolite metamorphic facies Lowlands, in the northwest, and the granulite facies Central Highlands, which are 86 THE ADIRONDACK JOURNAL OF ENVIRONMENTAL STUDIES VOLUME 21 87 7: MINING, GEOLOGY, AND GEOLOGICAL HISTORY OF GARNET AT THE BARTON GARNET MINE, GORE MOUNTAIN, NEW YORK separated by a very large, northwest-dipping fault zone. -

Contributions to Economic Geology, 1905
CONTRIBUTIONS TO ECONOMIC GEOLOGY, 1905. S. F. EMMONS, E. C. ECKEL, Geologists in Charge. INTRODUCTION. By C. W. HA YES, Geologist in Charge of Geology. This bulletin is the fourth of a series, including Bulletins Nos. 213, 225, and 260, Con tributions to Economic. Geology for 1902, 1903, and 1904, respectively. These bulletins are prepared primarily with a view to securing prompt publication of the economic results of investigations made by the United States Geological Survey. They are designed to meet the wants of the busy man, and are so condensed that he will bo able to obtain results and conclusions with a minimum expenditure of time and energy. They also afford a better idea of the work which the Survey.as an organization is carrying on for the direct advancement of mining interests throughout the country than can readily be obtained from the more voluminous reports. In the first two bulletins of this series were included numerous papers relating to the economic geology of Alaska. In view of the rapid increase of economic work both in Alaska and in the States and the organization-of a division of Alaskan mineral resource's, distinct from the division of geology, it was last year considered advisable to exclude all papers relating to Alaska. These were brought together in a separate volume entitled ".Report of Pi-ogress of Investigations of Mineral Resources of Alaska in 1904," Bulletin No. 259. A similar segregation of papers relating to Alaska has been made this year. In the preparation of the present volume promptness of publication has been made secondary only to the economic utility of the material presented. -
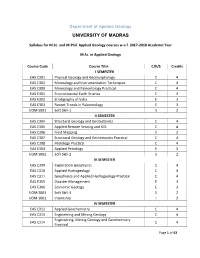
Dept of Applied Geology 28092017.1.1
Department of Applied Geology UNIVERSITY OF MADRAS Syllabus for M.Sc. and M.Phil. Applied Geology courses w.e.f. 2017-2018 Academic Year M.Sc. in Applied Geology Course Code Course Title C/E/S Credits I SEMESTER EAS C301 Physical Geology and Geomorphology C 4 EAS C302 Mineralogy and Instrumentation Techniques C 4 EAS C303 Mineralogy and Paleontology Practical C 4 EAS E301 Environmental Earth Science E 3 EAS E302 Stratigraphy of India E 3 EAS E303 Recent Trends in Paleontology E 3 UOM S001 Soft Skill-1 S 2 II SEMESTER EAS C304 Structural Geology and Geotectonics C 4 EAS C305 Applied Remote Sensing and GIS C 4 EAS C306 Field Mapping C 2 EAS C307 Structural Geology and Geotectonics Practical C 4 EAS C308 Petrology Practical C 4 EAS E304 Applied Petrology E 3 UOM S002 Soft Skill-2 S 2 III SEMESTER EAS C309 Exploration Geophysics C 4 EAS C310 Applied Hydrogeology C 4 EAS C311 Geophysics and Applied Hydrogeology Practical C 4 EAS E305 Disaster Management E 3 EAS E306 Economic Geology E 3 UOM S003 Soft Skill-3 S 2 UOM 1001 Internship I 2 IV SEMESTER EAS C312 Applied Geochemistry C 4 EAS C313 Engineering and Mining Geology C 4 Engineering, Mining Geology and Geochemistry EAS C314 C 4 Practical Page 1 of 43 EAS C315 Geological Field Tour C 2 EAS C316 Dissertation C 4 EAS E 307 Oceanography E 3 UOM S004 Soft Skill-4 S 2 ELECTIVES OFFERED TO OTHER DEPARTMENT STUDENTS EAS E308 Remote Sensing and GIS E 3 EAS E309 Climatology E 3 EAS E310 Environmental Geology E 3 EAS E311 Geological Oceanography E 3 I – SEMESTER EAS C 301 Physical Geology and Geomorphology C 3 1 0 4 Dr. -

Message from Our Interim Department Head, Dr. M. Stephen Enders
Colorado School of Mines College of Earth Resource Sciences and Engineering 2016 Department Newsletter Department of Geology and Geological Engineering Message from our Interim Department Head, Dr. M. Stephen Enders Welcome back to our Departmental Newsletter. The last issue was published in December 2014, and a lot of change occurred since then. For those of you wondering what happened, the Department went through quite a transition, and has come out even stronger than before and hell-bent on getting even better. This issue of the Newsletter focuses on news over the last year, and is still a work in pro- gress. There are probably some things you’d like to see us cover in subsequent newsletters, so please let us know. Ramona Graves, Dean of the College of Earth Resource Sciences and Engineering, asked me to take on the Interim Department Head position when Paul Santi stepped down to re-focus on his teaching and research interests in mid-May. Paul did an outstanding job lead- ing the Department through some rough times and re-establishing a healthy and invigorating culture. On behalf of the faculty and staff, I want to thank him again for all he has done for the Department and wish him great success in his teaching and research programs ahead. I am absolutely delighted to be the Interim Department Head. Some of you may know that I’m an alum of our Department having graduated with a BSc in 1976, and I’m honored to have been asked to take on the Department Head role. I have enjoyed a 40-year ca- reer in the mining business; and prior to joining the Department, I had been a Research Professor in the Mining Engineering Depart- ment with a joint appointment in GE since 2009.