Global Biogeography Since Pangaea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proterozoic East Gondwana: Supercontinent Assembly and Breakup Geological Society Special Publications Society Book Editors R
Proterozoic East Gondwana: Supercontinent Assembly and Breakup Geological Society Special Publications Society Book Editors R. J. PANKHURST (CHIEF EDITOR) P. DOYLE E J. GREGORY J. S. GRIFFITHS A. J. HARTLEY R. E. HOLDSWORTH A. C. MORTON N. S. ROBINS M. S. STOKER J. P. TURNER Special Publication reviewing procedures The Society makes every effort to ensure that the scientific and production quality of its books matches that of its journals. Since 1997, all book proposals have been refereed by specialist reviewers as well as by the Society's Books Editorial Committee. If the referees identify weaknesses in the proposal, these must be addressed before the proposal is accepted. Once the book is accepted, the Society has a team of Book Editors (listed above) who ensure that the volume editors follow strict guidelines on refereeing and quality control. We insist that individual papers can only be accepted after satis- factory review by two independent referees. The questions on the review forms are similar to those for Journal of the Geological Society. The referees' forms and comments must be available to the Society's Book Editors on request. Although many of the books result from meetings, the editors are expected to commission papers that were not pre- sented at the meeting to ensure that the book provides a balanced coverage of the subject. Being accepted for presentation at the meeting does not guarantee inclusion in the book. Geological Society Special Publications are included in the ISI Science Citation Index, but they do not have an impact factor, the latter being applicable only to journals. -

The Geologic Time Scale Is the Eon
Exploring Geologic Time Poster Illustrated Teacher's Guide #35-1145 Paper #35-1146 Laminated Background Geologic Time Scale Basics The history of the Earth covers a vast expanse of time, so scientists divide it into smaller sections that are associ- ated with particular events that have occurred in the past.The approximate time range of each time span is shown on the poster.The largest time span of the geologic time scale is the eon. It is an indefinitely long period of time that contains at least two eras. Geologic time is divided into two eons.The more ancient eon is called the Precambrian, and the more recent is the Phanerozoic. Each eon is subdivided into smaller spans called eras.The Precambrian eon is divided from most ancient into the Hadean era, Archean era, and Proterozoic era. See Figure 1. Precambrian Eon Proterozoic Era 2500 - 550 million years ago Archaean Era 3800 - 2500 million years ago Hadean Era 4600 - 3800 million years ago Figure 1. Eras of the Precambrian Eon Single-celled and simple multicelled organisms first developed during the Precambrian eon. There are many fos- sils from this time because the sea-dwelling creatures were trapped in sediments and preserved. The Phanerozoic eon is subdivided into three eras – the Paleozoic era, Mesozoic era, and Cenozoic era. An era is often divided into several smaller time spans called periods. For example, the Paleozoic era is divided into the Cambrian, Ordovician, Silurian, Devonian, Carboniferous,and Permian periods. Paleozoic Era Permian Period 300 - 250 million years ago Carboniferous Period 350 - 300 million years ago Devonian Period 400 - 350 million years ago Silurian Period 450 - 400 million years ago Ordovician Period 500 - 450 million years ago Cambrian Period 550 - 500 million years ago Figure 2. -

The New Global History Toward a Narrative for Pangaea Two
EWE (previously EuS) 14(2003)1 Main Article / Hauptartikel 75 The New Global History Toward a Narrative for Pangaea Two Wolf Schäfer Summary: The author approaches contemporary history from a position that defines the expression “truly global” as earth-centered. On this ground, two kinds of global history can be distinguished: the non-linear geophysical history of first nature, which is alternately uniting and dividing the world-continent (called Pangaea One), and the revolutionary technoscientific history of second nature, which is beginning to defragment the earth with things like the World Wide Web. The new Global History explores contemporary global history in general and the emerging civilizational unity (called Pangaea Two) in particular. The new historiographical approach is introduced via a critical discussion of Globality, Globalization, Periodization, World History, and Methodology. Zusammenfassung: Der Autor nähert sich der Gegenwartsgeschichte von einer Position, die den Ausdruck “wahrhaft global” als erdzentriert definiert. Auf dieser Basis können zwei Arten von Globalgeschichte unterschieden werden: die nicht-lineare geophysikalische Geschichte der ersten Natur, die den Weltkontinent abwechselnd zusammenfügt und fragmentiert (Pangaea Eins genannt), und die revolutionäre technowissenschaftliche Geschichte der zweiten Natur, die angefangen hat die Erde mit Dingen wie dem World Wide Web zu defragmentieren. Die neue Globalgeschichte erforscht die globale Gegenwartsgeschichte im allgemeinen und die sich anbahnende zivilisatorische Einheit (Pangaea Zwei genannt) im besonderen. Der neue historiographische Ansatz wird anhand einer kritischen Diskussion von Globalität, Globalisierung, Periodisierung, Weltgeschichte und Methodologie vorgestellt. Overview ((3)) In this article, I shall be following an alternative tack to- ward global history. I think that the designation “new global history” is warranted by the novelty of a human-made global ((1)) Titles of the form “The New –” invite the tested response: history. -

A Bight Is Born
Islands in the Stream 2002: Exploring Underwater Oases A Bight is Born FOCUS Limestone The origin and structure of the southeast coastline Taconic Orogeny and continental shelf (South Atlantic Bight) Microcontinent Collisions GRADE LEVEL Alleghenian Orogeny 9 -12 (Earth Science) Equator (may be adapted to 7th-8th grade Earth Science Piedmont Standards) Monadnocks Rift basins FOCUS QUESTION Erosion What is the South Atlantic Bight and how was it Coastal formed? Volcanic Sediment LEARNING OBJECTIVES Continental shelf Students will sequence and model the events lead- Regresses ing to the formation of the southeastern coast of the Ecosystems United States, including the formation of the South Atlantic Bight. The words listed as key words are integral to the unit. There are no formal signs in American Sign Students will examine what forces have changed Language for any of these words and many are the structure of this area over geologic time, pre- difficult to lipread. Having the vocabulary list on dict what changes are yet to occur and how these the board as a reference during the lesson will be changes have and may affect the populations living extremely helpful. This activity presents a great in this area, including man. deal of new material and requires a large amount of reading and attention. Many new words which ADDITIONAL INFORMATION FOR TEACHERS OF DEAF STUDENTS might be unfamiliar are included. This activity is In addition to the words listed as Key Words, the best conducted as a review or as an activity after following words should be part of the vocabulary the concepts have been taught. -
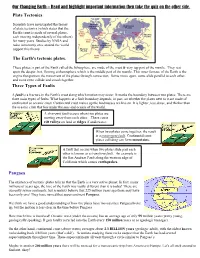
Plate Tectonics the Earth's Tectonic Plates. Three Types Of
Our Changing Earth – Read and highlight important information then take the quiz on the other side. Plate Tectonics Scientists have investigated the theory of plate tectonics (which states that the Earth's crust is made of several plates, each moving independently of the others) for many years. Studies by NASA and radio astronomy sites around the world support this theory. The Earth's tectonic plates. These plates, a part of the Earth called the lithosphere, are made of the crust & very top part of the mantle. They rest upon the deeper, hot, flowing asthenosphere which is the middle part of the mantle. This inner furnace of the Earth is the engine that powers the movement of the plates through convection. Some move apart, some slide parallel to each other, and some even collide and smash together. Three Types of Faults A fault is a fracture in the Earth's crust along which motion may occur. It marks the boundary between two plates. There are three main types of faults. What happens at a fault boundary depends, in part, on whether the plates next to it are made of continental or oceanic crust. Continental crust makes up the landmasses we live on. It is lighter, less dense, and thicker than the oceanic crust that lies under the seas and oceans of the world. A divergent fault occurs when two plates are moving away from each other. These cause rift valleys on land or ridges if underwater . When two plates come together, the result is a convergent fault. Continental crust plates colliding can form mountains. -

Earth History
Earth History Geography 106 LRS Doug Fischer Introduction – Overview of geologic history • Plate positions over time • Major biogeographic events Earth’s tectonic history • Gondwanaland – Southern continents – Formed 650mya Precambrian • Laurasia – Northern Continents – Most converged in Devonian 400mya as “old sandstone continent” • Formation of Pangaea – Late Permian ~ 275 mya Breakup of Pangaea • Started 180 mya (early Jurassic) – Prior to breakup, great mixing of biota – However, regionalization did still occur as it does on (smaller) continents today Breakup of Laurasia • Separated Europe & N. America 100 mya • Beringia rejoined them 75 mya • Intermittent connection via Greenland & Beringia through Tertiary Breakup of Gondwanaland • 180-160mya Gondwanaland started to split – Mesozoic (Triassic/Jurassic) • Mostly finished by 90 mya 152 mya 94 mya Central America and Antilles • Caribbean Plate was sandwiched between N&S America between 80 and 20 mya • Formed ring of islands • Landbridge closed ~ 3.5 mya – Great American Interchange 14 mya Biogeographic consequences of plate tectonics • Fragmentation and dispersal of ancestral biota (vicariance) • Changing barriers and coridors – biotic interchange • Speciation and extinction – changing physical and biological conditions Tour of Geologic History The geologic time scale • Phanerozoic starts with Cambrian explosion of species with hard body parts – (Some multi- cellular algae and animals lived at the end of the Precambrian) Paleozoic Paleozoic Cambrian • Animals with hard-shells appeared in great numbers for the first time • The continents were flooded by shallow seas. • The supercontinent of Gondwana had just formed and was located near the South Pole. Ordivician • ancient oceans separated the barren continents of Laurentia, Baltica, Siberia and Gondwana. • The end of the Ordovician was one of the coldest times in Earth history. -

GIS-Based Reconstruction of Pangaea with Recent Progresses in Plate Tectonics
GIS-based Reconstruction of Pangaea with Recent Progresses in Plate Tectonics O-hyeok Kwon1, Hyeong-Bin Cheong2, and Yang-Won Lee3 (Pukyong National University, Daeyean-3-dong Namgu, Busan 608-737, Korea) 1 Director, Institute for Regional Studies ([email protected]) 2 Professor, Department of Environmental Atmospheric Sciences 3 Professor, Department of Spatial Information Engineering I. Introduction □ There have been many debates about the existence and features of the supercontinent Pangaea since Alfred Wegener suggested the continental drift hypothesis. Now, the question about its existence was clearly solved. - Wegener’s proposed Pangaea matches well with the supercontinent reconstructed by modern scientists in the late 1960s based on concrete evidence and sophisticated scientific paradigms such as plate tectonics. □ Nonetheless, despite a wealth of geological data and the development of related areas of scientific enquiry, the precise shape of Pangaea before the supercontinent broke up and drifted apart has remained uncertain. - The Pangaean supercontinent proposed by Wegener is a huge rounded to oval landmass. Several other shapes for Pangaea are now recognized by the geological community. □ In this study, the shape of the Pangaean supercontinent was reconstructed incorporating recent advances in plate tectonics using a sophisticated geographical information system (GIS) mapping technique. - To better visualize of the shape of the supercontinent, Pangaea was reconstructed using an equidistant map projection where the central point of Pangaea was placed in the center of the map. □ Pangaea as reconstructed in this manner appears almost circular in shape with two large inland seas, unlike in previous reconstructions. 1 II. The Shape of Pangaea in Previous Studies 1. -

14-K. Yokoyama-4.02
国立科博専報,(45): 139–148, 2008年3月28日 Mem. Natl. Mus. Nat. Sci., Tokyo, (45): 139–148, March 28, 2008 Reconnaissance Study of Monazite Age from Southeast Asia Kazumi Yokoyama* and Yukiyasu Tsutsumi Department of Geology and Paleontology, National Museum of Nature and Science, 3–23–1 Hyakunin-cho, Shinjuku-ku, Tokyo 169–0073, Japan * Corresponding author: e-mail: [email protected] Abstract. Southeast Asia has a complex tectonic framework. The old craton, Indochina craton, is surrounded by Cenozoic accretional zone and magmatic arc. Many age data have been presented from the craton, though they have not been determined in a regional scale. We analyzed detrital monazites in the sands from the rivers cutting through the craton and surrounding younger ter- ranes. Peaks in the age distribution of monazites from the Mekong and Sap Rivers are in ranges of 200–300 Ma and 400–500 Ma. In the other areas, major peaks are less than 300 Ma. A small peak with 500–600 Ma was recognized from the river running through Thailand. Such an old monazite is thought to be derived from the nearby sub-Indian continent at the time of Pangaea age. Monazite with 110 Ma or younger peaks is due to the Cenozoic magmatic event at the time of subduction. The Triassic age, around 240 Ma, is attributed to the breakup of the Pangaea or events related to the collision of Yangtze and North China cratons. The oldest peak, 450 Ma, is a key age which should be present before the breakup event of the Pangaea. The age is not correlated with the age data of the India subcontinent, but is similar to that in the southern part of the Yangtze craton, sug- gesting that the Indochina craton was parentally formed with a part of the Yangtze craton. -

Supercontinent Reconstruction the Palaeomagnetically Viable, Long
Geological Society, London, Special Publications The palaeomagnetically viable, long-lived and all-inclusive Rodinia supercontinent reconstruction David A. D. Evans Geological Society, London, Special Publications 2009; v. 327; p. 371-404 doi:10.1144/SP327.16 Email alerting click here to receive free email alerts when new articles cite this service article Permission click here to seek permission to re-use all or part of this article request Subscribe click here to subscribe to Geological Society, London, Special Publications or the Lyell Collection Notes Downloaded by on 21 December 2009 © 2009 Geological Society of London The palaeomagnetically viable, long-lived and all-inclusive Rodinia supercontinent reconstruction DAVID A. D. EVANS Department of Geology & Geophysics, Yale University, New Haven, CT 06520-8109, USA (e-mail: [email protected]) Abstract: Palaeomagnetic apparent polar wander (APW) paths from the world’s cratons at 1300–700 Ma can constrain the palaeogeographic possibilities for a long-lived and all-inclusive Rodinia supercontinent. Laurentia’s APW path is the most complete and forms the basis for super- position by other cratons’ APW paths to identify possible durations of those cratons’ inclusion in Rodinia, and also to generate reconstructions that are constrained both in latitude and longitude relative to Laurentia. Baltica reconstructs adjacent to the SE margin of Greenland, in a standard and geographically ‘upright’ position, between c. 1050 and 600 Ma. Australia reconstructs adja- cent to the pre-Caspian margin of Baltica, geographically ‘inverted’ such that cratonic portions of Queensland are juxtaposed with that margin via collision at c. 1100 Ma. Arctic North America reconstructs opposite to the CONgo þ Sa˜o Francisco craton at its DAmaride–Lufilian margin (the ‘ANACONDA’ fit) throughout the interval 1235–755 Ma according to palaeomag- netic poles of those ages from both cratons, and the reconstruction was probably established during the c. -

How Subduction Broke up Pangaea with Implications for the Supercontinent Cycle
Downloaded from http://sp.lyellcollection.org/ by guest on September 30, 2021 How subduction broke up Pangaea with implications for the supercontinent cycle FRASER KEPPIE Department of Energy, Government of Nova Scotia, Joseph Howe Building, 12th Floor, 1690 Hollis Street, Halifax, Nova Scotia, B3J 3J9, Canada (e-mail: [email protected]) Abstract: Mechanisms that can explain the Mesozoic motion of Pangaea in a palaeomagne- tic mantle reference frame may also be able to explain its breakup. Calculations indicate that Pangaea moved along a non-rigid path in the mantle frame between the late Triassic and early Jurassic. The breakup of Pangaea may have happened as a response to this non-rigid motion. Tec- tonic forces applied to the margins of Pangaea as a consequence of subduction at its peripheries can explain both the motion and deformation of Pangaea with a single mechanism. In contrast, mantle forces applied to the base of Pangaea appear to be inconsistent with the kinematic con- straints and do not explain the change in supercontinent motion that accompanied the breakup event. Top-down plate tectonics are inferred to have caused the breakup of Pangaea. Strong coup- ling between the mantle and lithosphere may not have been the case during the Phanerozoic eon when the Pangaean supercontinent formed and subsequently dispersed. Gold Open Access: This article is published under the terms of the CC-BY 3.0 license. The mechanisms responsible for plate tectonic analysis builds on the previous work of D. F. change on Earth have been linked to the different Keppie (2015) in which only the relative plate stages of the supercontinent cycle (Worsley et al. -

PUBLIC NOTICE FEDERAL COMMUNICATIONS COMMISSION 445 12Th STREET S.W
PUBLIC NOTICE FEDERAL COMMUNICATIONS COMMISSION 445 12th STREET S.W. WASHINGTON D.C. 20554 News media information 202-418-0500 Internet: http://www.fcc.gov (or ftp.fcc.gov) TTY (202) 418-2555 DA No. 19-888 Report No. SCL-00250 Monday September 9, 2019 Actions Taken Under Cable Landing License Act Section 1.767(a) Cable Landing Licenses, Modifications, and Assignments or Transfers of Control of Interests in Cable Landing Licenses (47 C.F.R. § 1.767(a)) By the Chief, Telecommunications and Analysis Division, International Bureau: Pursuant to An Act Relating to the Landing and Operation of Submarine Cables in the United States, 47 U.S.C. §§ 34-39 (Cable Landing License Act), Executive Order No. 10530, Exec. Ord. No. 10530 reprinted as amended in 3 U.S.C. § 301, and section 1.767 of the Commission's rules, 47 C.F.R. § 1.767, the following applications ARE GRANTED. These grants of authority are taken under section 0.261 of the Commission's rules, 47 C.F.R. § 0.261. Petitions for reconsideration under section 1.106 or applications for review under section 1.115 of the Commission's rules, 47 C.F.R. §§ 1.106, 1.115, may be filed within 30 days of the date of this public notice. These applications have been coordinated with the Department of State and other Executive Branch agencies pursuant to section 1.767(b) of the Commission's rules, 47 C.F.R. §1.767(b), and consistent with procedures established with the Department of State. See Review of Commission Consideration of Applications under the Cable Landing License Act, IB Docket No. -

Fun Facts About the Dinosaurs of Gondwana
FUN FACTS ABOUT THE DINOSAURS OF GONDWANA Gondwana was a supercontinent comprising the landmasses of Africa, South America, Australia, and Antarctica, as well as Madagascar and India. It formed when the dynamic forces of plate tectonics split the larger supercontinent of Pangaea in two about 150 million years ago, and it lasted until about 100 million years ago, when it fractured into the individual continents of the Southern Hemisphere. Recent research shows that Gondwana was home to an almost entirely different set of dinosaurs that evolved in their own unique ways, largely isolated from the dinosaurs we’re more familiar with in the Northern Hemisphere. Thirty years ago, many of these species—including Giganotosaurus, Spinosaurus, and Argentinosaurus—were unknown to science. Eoraptor, one of the species from Argentina that is featured in Ultimate Dinosaurs, was one of the world’s earliest dinosaurs. It was small, for a dinosaur—only three feet in length and weighed approximately 25 pounds. Fossilized remains of Suchomimus were first uncovered in the Sahara Desert of Niger in 1997. This animal had a long snout like a crocodile that allowed it to catch fish. In fact, its name means “crocodile mimic.” The skulls of spinosaurs like Suchomimus and crocodiles represent an example of convergent evolution, or the acquisition of similar biological traits in response to similar ecological challenges. The plant-eating Ouranosaurus from central Africa had a row of spines along its back that formed a sail. Paleontologists debate the function of this feature. Was it for body temperature regulation? Storing nutrients and fat? Protection? Attracting mates? Bones of Majungasaurus with Majungasaurus tooth marks show that this meat-eating theropod dinosaur from Madagascar was a cannibal, at least some of the time.