IDENTIFYING OAKS: the HYBRID PROBLEM by Richard J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New York City Approved Street Trees
New York City Approved Street Trees Suggested Tree Species Shape Visual interest Frequency of Preferred Cultivars Notes Scientific Name Common Name Planting Acer rubrum Red Maple Sparingly 'Red Sunset' ALB Host Aesculus hippocastanum Horsechestnut White May flowers Sparingly 'Baumanni' ALB Host Aesculus octandra Yellow Buckeye Yellow May Flowers Sparingly ALB Host ALB Host 'Duraheat' Betula nigra River Birch Ornamental Bark Sparingly Plant Single Stem 'Heritage' Only Celtis occidentalis Hackberry Ornamental Bark Sparingly 'Magnifica' ALB Host ALB Host Cercidiphyllum japonicum Katsura Tree Sparingly Plant Single Stem Only Corylus colurna Turkish Filbert Sparingly LARGE TREES: Mature LARGE TREES: height than greater feet 50 tall Eucommia ulmoides Hardy Rubber Tree Frequently 'Asplenifolia' Fagus sylvatica European Beech Sparingly 'Dawyckii Purple' 'Autumn Gold' Ginkgo biloba Ginkgo Yellow Fall Color Moderately 'Magyar' Very Tough Tree 'Princeton Sentry' 'Shademaster' 'Halka' Gleditsia triacanthos var inermis Honeylocust Yellow Fall Color Moderately 'Imperial' 'Skyline' 'Espresso' Gymnocladus dioicus Kentucky Coffeetree Large Tropical Leaves Frequently 'Prairie Titan' Page 1 of 7 New York City Approved Street Trees Suggested Tree Species Shape Visual interest Frequency of Preferred Cultivars Notes Scientific Name Common Name Planting 'Rotundiloba' Seedless Cultivars Liquidambar styraciflua Sweetgum Excellent Fall Color Frequently 'Worplesdon' Preffered 'Cherokee' Orange/Green June Liriodendron tulipifera Tulip Tree Moderately Flowers Metasequoia -

Checklist of Illinois Native Trees
Technical Forestry Bulletin · NRES-102 Checklist of Illinois Native Trees Jay C. Hayek, Extension Forestry Specialist Department of Natural Resources & Environmental Sciences Updated May 2019 This Technical Forestry Bulletin serves as a checklist of Tree species prevalence (Table 2), or commonness, and Illinois native trees, both angiosperms (hardwoods) and gym- county distribution generally follows Iverson et al. (1989) and nosperms (conifers). Nearly every species listed in the fol- Mohlenbrock (2002). Additional sources of data with respect lowing tables† attains tree-sized stature, which is generally to species prevalence and county distribution include Mohlen- defined as having a(i) single stem with a trunk diameter brock and Ladd (1978), INHS (2011), and USDA’s The Plant Da- greater than or equal to 3 inches, measured at 4.5 feet above tabase (2012). ground level, (ii) well-defined crown of foliage, and(iii) total vertical height greater than or equal to 13 feet (Little 1979). Table 2. Species prevalence (Source: Iverson et al. 1989). Based on currently accepted nomenclature and excluding most minor varieties and all nothospecies, or hybrids, there Common — widely distributed with high abundance. are approximately 184± known native trees and tree-sized Occasional — common in localized patches. shrubs found in Illinois (Table 1). Uncommon — localized distribution or sparse. Rare — rarely found and sparse. Nomenclature used throughout this bulletin follows the Integrated Taxonomic Information System —the ITIS data- Basic highlights of this tree checklist include the listing of 29 base utilizes real-time access to the most current and accept- native hawthorns (Crataegus), 21 native oaks (Quercus), 11 ed taxonomy based on scientific consensus. -

Designing Hardwood Tree Plantings for Wildlife Brian J
FNR-213 Hardwood Tree Improvement and Regeneration Center North Central Research Station USDA Forest Service Department of Forestry and Natural Resources Purdue University Designing Hardwood Tree Plantings for Wildlife Brian J. MacGowan, Department of Forestry and Natural Resources, Purdue University Woody plants can be of value to many wildlife species. The species of tree or shrub, or the location, size, and shape of planting can all have an impact on wildlife. The purpose of this paper is to discuss the benefits of trees and shrubs for wildlife and how to design tree and shrub plantings for wildlife. Some of the practices may conflict with other management goals and may have to be modified for individual priorities. Trees and Shrubs for Wildlife The species you select for a tree planting should depend on the growing conditions of the site and the wildlife species that you want to manage. Talk to a professional forester to help you select the tree species best suited for your growing conditions. A professional biologist, such as a Department of Natural Resources District Biologist (www.in.gov/ food source for wildlife (Table 2). Shrubs can be dnr/fishwild/huntguide1/wbiolo.htm), can assist you particularly important because several species of with planning a tree planting for wildlife. wildlife, especially songbirds, prefer to feed or nest There is no specific formula for developing wild- on or near the ground. Shrubs also provide good life habitat. For example, acorns are eaten by a wide protective cover for these types of wildlife. Pines variety of wildlife species including tree squirrels, and other softwoods provide limited food, but are an pheasants, wild turkey, and deer. -

Quercus Imbricaria.Indd
Quercus imbricaria (Shingle Oak) Beech Family (Fagaceae) Introduction: Shingle oak is a member of the red oak group with willow-like leaves. It is one of the most handsome of the oaks. Shingle oak has an attractive branching habit and ridged bark, and it casts medium shade in summer. Although fall color may not be outstanding, the shiny, willow-shaped leaves are nonetheless quite attractive through all four seasons. Culture: Shingle oak is an easy oak to grow and adapts to various sites. While it prefers rich, moist, acidic soil and full sun, it is tolerant of drought, urban conditions and slightly alkaline soil. Shingle oak is easy to trans- plant. Because of its very strong wood, this oak is not Botanical Characteristics: subject to storm damage. Shingle oak has few serious insect and disease Native habitat: Central and eastern North problems, although potential problems include obscure America in rich woods. scale, two-lined chestnut borer, bacterial leaf scorch, oak horn gall and gypsy moth. In addition, as little as 1 Growth habit: The tree is pyramidal when inch of fi ll soil can kill an oak. young but becomes wide-spreading with matu- rity. Additional information: Shingle oak may be one of the best oaks, but it Tree size: Shingle oak will slowly attain a height of 50 to 60 feet with a similar or greater is not commonly used. It makes a good park or street spread. It can reach 100 feet tall in the wild. tree. Because it adapts to pruning and has persistent leaves, it is useful as a hedge. -

Key to Leaves of Eastern Native Oaks
FHTET-2003-01 January 2003 Front Cover: Clockwise from top left: white oak (Q. alba) acorns; willow oak (Q. phellos) leaves and acorns; Georgia oak (Q. georgiana) leaf; chinkapin oak (Q. muehlenbergii) acorns; scarlet oak (Q. coccinea) leaf; Texas live oak (Q. fusiformis) acorns; runner oak (Q. pumila) leaves and acorns; background bur oak (Q. macrocarpa) bark. (Design, D. Binion) Back Cover: Swamp chestnut oak (Q. michauxii) leaves and acorns. (Design, D. Binion) FOREST HEALTH TECHNOLOGY ENTERPRISE TEAM TECHNOLOGY TRANSFER Oak Identification Field Guide to Native Oak Species of Eastern North America John Stein and Denise Binion Forest Health Technology Enterprise Team USDA Forest Service 180 Canfield St., Morgantown, WV 26505 Robert Acciavatti Forest Health Protection Northeastern Area State and Private Forestry USDA Forest Service 180 Canfield St., Morgantown, WV 26505 United States Forest FHTET-2003-01 Department of Service January 2003 Agriculture NORTH AMERICA 100th Meridian ii iii ACKNOWLEDGMENTS The authors wish to thank all those who helped with this publication. We are grateful for permission to use the drawings illustrated by John K. Myers, Flagstaff, AZ, published in the Flora of North America, North of Mexico, vol. 3 (Jensen 1997). We thank Drs. Cynthia Huebner and Jim Colbert, U.S. Forest Service, Northeastern Research Station, Disturbance Ecology and Management of Oak-Dominated Forests, Morgantown, WV; Dr. Martin MacKenzie, U.S. Forest Service, Northeastern Area State and Private Forestry, Forest Health Protection, Morgantown, WV; Dr. Steven L. Stephenson, Department of Biology, Fairmont State College, Fairmont, WV; Dr. Donna Ford-Werntz, Eberly College of Arts and Sciences, West Virginia University, Morgantown, WV; Dr. -

Ouachita Mountains Ecoregional Assessment December 2003
Ouachita Mountains Ecoregional Assessment December 2003 Ouachita Ecoregional Assessment Team Arkansas Field Office 601 North University Ave. Little Rock, AR 72205 Oklahoma Field Office 2727 East 21st Street Tulsa, OK 74114 Ouachita Mountains Ecoregional Assessment ii 12/2003 Table of Contents Ouachita Mountains Ecoregional Assessment............................................................................................................................i Table of Contents ........................................................................................................................................................................iii EXECUTIVE SUMMARY..............................................................................................................1 INTRODUCTION..........................................................................................................................3 BACKGROUND ...........................................................................................................................4 Ecoregional Boundary Delineation.............................................................................................................................................4 Geology..........................................................................................................................................................................................5 Soils................................................................................................................................................................................................6 -

Morristown Street Tree Resource Booklet
Morristown Street Tree Resource Booklet June 2020 I. Large Shade Trees for Areas Larger than 4’ x 6’ 3 Black Tupelo (Nyssa sylcatica) 4 Dawn Redwood (Metasequoia glyptostroboides) 5 Elm (Ulmus spp.) 6 Gingko (Gingko biloba) 7 Hardy Rubber Tree (Eucommia ulmoides) 8 Honey Locust (Gleditsia triacanthos inermis) 9 Katsura Tree (Cercidphyllum japonicum) 10 Kentucky Coffee Tree (Gymnocladus dioicus) 11 Linden (Tilia spp) 12 Little Leaf Linden (Tilia cordata) 13 Silver Linden (Tilia tomentosa) 14 Crimean Linden (Tilia x euchlora) 15 London Plane Tree (Platanus x acerfolia) 16 Maple, Red (Acer rubrum) 17 Maple, Sugar ( Acer saccharum) 18 Oak, Pin (Quercus palustris) 19 Oak, Red (Quercus rubra) 20 Oak, Shingle (Quercus imbricaria) 21 Oak, White (Quercus alba) 22 Oak, Willow (Quercus phellos) 23 Pagoda Tree (Styphnolobium japanicum) 24 Sweetgum (Liquidambur styraciflua) 25 Japanese Zelkova (Zelkova serrata) 26 II. Understory Small and Medium Trees for Areas Larger than 2’ x 6’ 27 American Yellowwood (Cladrastis kentukea) 28 Amur Maackia (Maackia amurensis) 29 Cherry (Prunus spp) 30 Crabapple (Malus spp) 31 Dogwood (Cornus spp) 32 Eastern Rudbud (Cercis canadensis) 33 Golden Raintree (Koelreuteria paniculata) 34 Hackberry (Celtis occidentalis) 35 Hawthorne (Crataegus spp) 36 Hop Hornbeam (Ostrya virginiana) 37 Japanese Snowball (Styrax japonicas) 38 Maple Amur (Acer ginnala ‘Flame’) 39 Maple, Hedge (Acer campestre) 40 Purpleleaf Plum (Prunus cerasifera) 41 Callery Pear (Pyrus calleryanan’) 42 I. Large Shade Trees for Areas Larger than 4’ x 6’ Black Tupelo (Nyssa sylcatica) Form: Pyramidal in youth with horizontal branches forming, and rounded or irregular crown. Mature Height: 30’ to 50’ Mature Spread: 20’ to 30’ Use: Acceptable street tree. -

2019 Oklahoma Native Plant Record
30 Oklahoma Native Plant Record Volume 19, December 2019 A WALK THROUGH THE McLOUD HIGH SCHOOL OAK-HICKORY FOREST WITH A CHECKLIST OF THE WOODY PLANTS Bruce A. Smith McLoud High School 1100 West Seikel McLoud, OK 74851 [email protected] ABSTRACT The McLoud High School oak-hickory forest is located a short distance from the McLoud High School campus. The forest has been used as an outdoor classroom for many years for high school students. This article will guide you through the forest trail and discuss several woody plants of interest at 16 landmarks. The article also includes a checklist of the 38 woody species identified in the forest. Key words: woody plants, checklist, invasive plants, hybridization, leaf curl INTRODUCTION indicated, all photos were taken by McLoud High School Botany classes over a number The McLoud High School forest has of years. been an important element in my teaching career for many years. I can’t remember the STUDY AREA first time that we started using the McLoud oak-hickory forest as an outdoor classroom. The forest is about 100 m (330 ft) by I do remember Kari Courkamp doing 76 m (250 ft) and is located near the research on tree lichens 25 years ago. Since McLoud High School campus in McLoud, that time there was a long period when we Oklahoma. It is bordered by adjacent used it mostly to learn about the forests on the south and east. The forest has composition and structure of the forest. In been utilized as an outdoor classroom for the last few years, we have done a variety of the high school students for many years. -
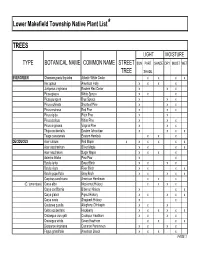
Native Plant List Trees.XLS
Lower Makefield Township Native Plant List* TREES LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE EVERGREEN Chamaecyparis thyoides Atlantic White Cedar x x x x IIex opaca American Holly x x x x Juniperus virginiana Eastern Red Cedar x x x Picea glauca White Spruce x x x Picea pungens Blue Spruce x x x Pinus echinata Shortleaf Pine x x x Pinus resinosa Red Pine x x x Pinus rigida Pitch Pine x x Pinus strobus White Pine x x x Pinus virginiana Virginia Pine x x x Thuja occidentalis Eastern Arborvitae x x x x Tsuga canadensis Eastern Hemlock xx x DECIDUOUS Acer rubrum Red Maple x x x x x x Acer saccharinum Silver Maple x x x x Acer saccharum Sugar Maple x x x x Asimina triloba Paw-Paw x x Betula lenta Sweet Birch x x x x Betula nigra River Birch x x x x Betula populifolia Gray Birch x x x x x Carpinus caroliniana American Hornbeam x x x (C. tomentosa) Carya alba Mockernut Hickory x x x x Carya cordiformis Bitternut Hickory x x x Carya glabra Pignut Hickory x x x x x Carya ovata Shagbark Hickory x x Castanea pumila Allegheny Chinkapin xx x Celtis occidentalis Hackberry x x x x x x Crataegus crus-galli Cockspur Hawthorn x x x x Crataegus viridis Green Hawthorn x x x x Diospyros virginiana Common Persimmon x x x x Fagus grandifolia American Beech x x x x PAGE 1 Exhibit 1 TREES (cont'd) LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE DECIDUOUS (cont'd) Fraxinus americana White Ash x x x x Fraxinus pennsylvanica Green Ash x x x x x Gleditsia triacanthos v. -

An Updated Infrageneric Classification of the North American Oaks
Article An Updated Infrageneric Classification of the North American Oaks (Quercus Subgenus Quercus): Review of the Contribution of Phylogenomic Data to Biogeography and Species Diversity Paul S. Manos 1,* and Andrew L. Hipp 2 1 Department of Biology, Duke University, 330 Bio Sci Bldg, Durham, NC 27708, USA 2 The Morton Arboretum, Center for Tree Science, 4100 Illinois 53, Lisle, IL 60532, USA; [email protected] * Correspondence: [email protected] Abstract: The oak flora of North America north of Mexico is both phylogenetically diverse and species-rich, including 92 species placed in five sections of subgenus Quercus, the oak clade centered on the Americas. Despite phylogenetic and taxonomic progress on the genus over the past 45 years, classification of species at the subsectional level remains unchanged since the early treatments by WL Trelease, AA Camus, and CH Muller. In recent work, we used a RAD-seq based phylogeny including 250 species sampled from throughout the Americas and Eurasia to reconstruct the timing and biogeography of the North American oak radiation. This work demonstrates that the North American oak flora comprises mostly regional species radiations with limited phylogenetic affinities to Mexican clades, and two sister group connections to Eurasia. Using this framework, we describe the regional patterns of oak diversity within North America and formally classify 62 species into nine major North American subsections within sections Lobatae (the red oaks) and Quercus (the Citation: Manos, P.S.; Hipp, A.L. An Quercus Updated Infrageneric Classification white oaks), the two largest sections of subgenus . We also distill emerging evolutionary and of the North American Oaks (Quercus biogeographic patterns based on the impact of phylogenomic data on the systematics of multiple Subgenus Quercus): Review of the species complexes and instances of hybridization. -

Juniperus Virginiana- Wiiqpedia Juniperus Virginiana
AGENDA EAST GOSHEN TOWNSHIP CONSERVANCY BOARD MEETING February 12, 2020- 7:00 PM 1. CALL TO ORDER/ PLEDGE OF ALLEGIANCE/ MOMENT OF SILENCE 2. APPROVAL OF MINUTES January 8, 2020 3. CHAIRMAN'S REPORT 4. OLD BUSINESS • 2020 Keep East Goshen Beautiful Day • 2020 Spring Event • Discuss 2020 goals and ABC Planning Meeting • East Goshen Ponds status (Marydel! and Bow Tree) 5. SUB DIVISION REVIEW 6. NEW BUSINESS • New Conservancy Board Member Appointments, Daniel Flynn & Leo Sinclair • Tree and Street Tree Resolution 7. VARIANCES/CONDITIONAL USES 8. BOARD MEMBER CONCERNS 9. LIAISON REPORTS 10. CORRESPONDENCE • Letter-January2 7, Zoning Hearing Board Notice 11. DATES OF IMPORTANCE Feb 13, 2020 Historical Commission 07:00pm Feb 17, 2020 Township Office Closed Feb 17, 2020 Futurist Committee 07:00pm Feb 18, 2020 Board of Supervisors 07:00pm Feb 24, 2020 Sustainability Advisory Committee 07:00pm Feb 27, 2020 Pipeline Task Force 05:00pm Mar 03, 2020 Board of Supervisors 07:00pm Mar 04, 2020 Planning Commission 07:00pm Mar 05, 2020 Park & Rec Commission 07:00pm Mar 09, 2020 Municipal Authority 07:00pm 12. PUBLIC COMMENT 13. ADJOURNMENT F:\Data\Shared Data\Agendas\Conservancy Board\2020\2020-02-l2_Conservancy Board Agenda.doc 1 DRAFT 2 EAST GOSHEN TOWNSHIP 3 CONSERVANCY BOARD MEETING 4 January 8, 2020 5 6 The East Goshen Township Conservancy Board held a regularly scheduled meeting on 7 Wednesday, January 8, 2020 at 7:00 p.m. at the Township Building. Members in attendance 8 are indicated in BOLD: 9 Chairman Walter Wujcik 10 Vice Chairman Andy Tyler 11 Erich Meyer 12 Sandra Snyder 13 Dan Flynn, new member 14 Others present were: 15 Michele Truitt, Township Supervisor 16 17 Call to Order 18 Walter called the meeting to order at 7:00 p.m. -

Ecological Site F116AY044MO Chert Dolomite Upland Woodland
Natural Resources Conservation Service Ecological site F116AY044MO Chert Dolomite Upland Woodland Last updated: 9/24/2020 Accessed: 09/30/2021 General information Figure 1. Mapped extent Areas shown in blue indicate the maximum mapped extent of this ecological site. Other ecological sites likely occur within the highlighted areas. It is also possible for this ecological site to occur outside of highlighted areas if detailed soil survey has not been completed or recently updated. MLRA notes Major Land Resource Area (MLRA): 116A–Ozark Highland The Ozark Highland constitutes the Salem Plateau of the Ozark Uplift. Elevation ranges from about 300 feet on the southeast edge of the Ozark escarpment, to about 1,600 feet in the west, adjacent to the Burlington Escarpment of the Springfield Plateau. The underlying bedrock is mainly horizontally bedded Ordovician-aged dolomites and sandstones that dip gently away from the uplift apex in southeast Missouri. Cambrian dolomites are exposed on deeply dissected hillslopes. In some places, Pennsylvanian and Mississipian sediments overlie the plateau. Relief varies, from the gently rolling central plateau areas to deeply dissected hillslopes associated with drainageways such as the Buffalo, Current, Eleven Point and White Rivers. Classification relationships Terrestrial Natural Community Type in Missouri (Nelson, 2010): The reference state for this ecological site is most similar to a Dry Chert Woodland. Missouri Department of Conservation Forest and Woodland Communities (MDC, 2006): The reference state for this ecological site is most similar to Post Oak Woodland. National Vegetation Classification System Vegetation Association (NatureServe, 2010): The reference state for this ecological site is most similar to Quercus stellata - Quercus marilandica - Quercus velutina - Carya texana / Schizachyrium scoparium Woodland (CEGL002149).