Structural Basis of Mammalian Mucin Processing by the Human Gut O
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Prokaryotes (Domains Bacteria & Archaea)
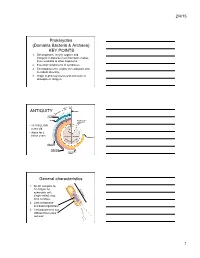
2/4/15 Prokaryotes (Domains Bacteria & Archaea) KEY POINTS 1. Decomposers: recycle organic and inorganic molecules in environment; makes them available to other organisms. 2. Essential components of symbioses. 3. Encompasses the origins of metabolism and metabolic diversity. 4. Origin of photosynthesis and formation of atmospheric Oxygen Ceno- Meso- zoic zoic ANTIQUITY Humans Paleozoic Colonization of land Animals Origin of solar system and Earth • >3.5 BILLION years old. • Alone for 2 1 4 billion years Proterozoic Archaean Prokaryotes Billions of 2 years ago3 Multicellular eukaryotes Single-celled eukaryotes Atmospheric oxygen General characteristics 1. Small: compare to 10-100µm for 0.5-5µm eukaryotic cell; single-celled; may form colonies. 2. Lack membrane- enclosed organelles. 3. Cell wall present, but different from plant cell wall. 1 2/4/15 General characteristics 4. Occur everywhere, most numerous organisms. – More individuals in a handful of soil then there are people that have ever lived. – By far more individuals in our gut than eukaryotic cells that are actually us. General characteristics 5. Metabolic diversity established nutritional modes of eukaryotes. General characteristics 6. Important decomposers and recyclers 2 2/4/15 General characteristics 6. Important decomposers and recyclers • Form the basis of global nutrient cycles. General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • About 1/2 of all human diseases are caused by Bacteria General characteristics 7. Symbionts!!!!!!! • Parasites • Pathogenic organisms. • Extremely important in agriculture as well. Pierce’s disease is caused by Xylella fastidiosa, a Gamma Proteobacteria. It causes over $56 million in damage annually in California. That’s with $34 million spent to control it! = $90 million in California alone. -

Wellcome Trust Annual Report and Financial Statements 2017 Contents
Annual Report and Financial Statements 2017 2 Wellcome Trust Annual Report and Financial Statements 2017 Contents Report from the Chair and the Director 5 Trustee’s Report 8 What we do 8 Review of Charitable Activities 9 Review of Investment Activities 18 Financial Review 29 Structure and Governance 34 Risk Management 37 Remuneration Report 40 Audit Committee Report 43 Independent Auditor’s Report 45 Financial Statements 58 Consolidated Statement of Financial Activities 58 Consolidated Balance Sheet 59 Statement of Financial Activities of the Trust 60 Balance Sheet of the Trust 61 Consolidated Cash Flow Statement 62 Notes to the Financial Statements 63 Reference and Administrative Details 117 3 Wellcome Trust Annual Report and Financial Statements 2017 “ At Wellcome, we believe in the power of ideas to improve health” Jeremy Farrar Director 4 Wellcome Trust Annual Report and Financial Statements 2017 Report from the Chair and the Director “Our core approach is funding people to explore great ideas, at every step of the way from discovery to impact” At Wellcome, we believe in the power of ideas to improve cause of maternal mortality in the world. It also includes health. Funded from our independent investment portfolio, supporting research in the humanities and social sciences, we support thousands of scientists and researchers in more such as a project which this year published ethical guidelines than 70 countries, as well as innovators, educators and artists. for involving pregnant women in Zika vaccine research. Together, we take on big problems, fuel imaginations and spark And resources like the Human Induced Pluripotent Stem Cell debate, working always to achieve better health for everyone. -

The Electron Microscopy Science Technology Platform at the Francis
The Francis Crick Institute is a The Electron biomedical discovery institute Microscopy dedicated to understanding the fundamental biology underlying Science health and disease. Its work Technology is helping to understand why disease develops and to translate Platform at discoveries into new ways to the Francis prevent, diagnose and treat illnesses such as cancer, heart Crick Institute disease, stroke, infections, and Lucy Collinson neurodegenerative diseases. An independent organisation, its founding partners are the Medical Research Council (MRC), Cancer Research UK, Wellcome, University College London, Imperial College London and King’s College London. The Crick was formed in 2015, with many of the Crick’s scientists joining from two ‘parent’ institutes, the MRC’s National Institute for Medical Research and Cancer Research UK’s London Research Institute, and in 2016 it moved into a brand new state-of- the-art building in central London which brings together 1500 scientists and support staff working collaboratively across disciplines, making it the biggest biomedical research facility under a single roof in Europe. © Nick Guttridge 4 ISSUE 46 JUNE 2017 5 Each microscope room is a six-sided shielded box, • The Phenom-World DelPhi benchtop SEM with walls that contain complex metallic layers has an integrated fluorescence microscope to attenuate DC fields, and an active cancellation for correlative imaging system to attenuate AC fields. Under each • The FEI Twin 120 kV TEM has a cryo stage for microscope is a concrete platform, cast in place, and screening vitrified macromolecular samples supported by air springs that remove environmental prior to imaging on 200 kV and 300 kV TEMs vibration to <1 Hz. -

A Generic Large-Scale Cause for Platelet Dysfunction and Depletion in Infection
Published online: 2020-04-12 THIEME 302 A Champion of Host Defense: A Generic Large-Scale Cause for Platelet Dysfunction and Depletion in Infection Martin J. Page, BSc (Hons)1 Etheresia Pretorius, PhD1 1 Department of Physiological Sciences, Stellenbosch University, Address for correspondence Etheresia Pretorius, PhD, Department of Stellenbosch, South Africa Physiological Sciences, Faculty of Science, Stellenbosch University, Private Bag X1 Matieland, Stellenbosch, 7602, South Africa Semin Thromb Hemost 2020;46:302–319. (e-mail: [email protected]). Abstract Thrombocytopenia is commonly associated with sepsis and infections, which in turn are characterized by a profound immune reaction to the invading pathogen. Platelets are one of the cellular entities that exert considerable immune, antibacterial, and antiviral actions, and are therefore active participants in the host response. Platelets are sensitive to surrounding inflammatory stimuli and contribute to the immune response by multiple mechanisms, including endowing the endothelium with a proinflammatory phenotype, enhancing and amplifying leukocyte recruitment and inflammation, promoting the effector functions of immune cells, and ensuring an optimal adaptive immune response. During infection, pathogens and their products influence the platelet response and can even be toxic. However, platelets are able to sense and engage bacteria and viruses to assist in their removal and destruction. Platelets greatly contribute to host defense by multiple mechanisms, including forming immune complexes and aggregates, shedding their granular content, and internalizing pathogens and subsequently being marked for removal. These processes, and the nature of platelet function in general, cause the platelet to be irreversibly consumed in Keywords the execution of its duty. An exaggerated systemic inflammatory response to infection ► platelets can drive platelet dysfunction, where platelets are inappropriately activated and face ► virus immunological destruction. -

Structure of Human Aspartyl Aminopeptidase Complexed With
Chaikuad et al. BMC Structural Biology 2012, 12:14 http://www.biomedcentral.com/1472-6807/12/14 RESEARCH ARTICLE Open Access Structure of human aspartyl aminopeptidase complexed with substrate analogue: insight into catalytic mechanism, substrate specificity and M18 peptidase family Apirat Chaikuad1, Ewa S Pilka1, Antonio De Riso2, Frank von Delft1, Kathryn L Kavanagh1, Catherine Vénien-Bryan2, Udo Oppermann1,3 and Wyatt W Yue1* Abstract Backround: Aspartyl aminopeptidase (DNPEP), with specificity towards an acidic amino acid at the N-terminus, is the only mammalian member among the poorly understood M18 peptidases. DNPEP has implicated roles in protein and peptide metabolism, as well as the renin-angiotensin system in blood pressure regulation. Despite previous enzyme and substrate characterization, structural details of DNPEP regarding ligand recognition and catalytic mechanism remain to be delineated. Results: The crystal structure of human DNPEP complexed with zinc and a substrate analogue aspartate-β- hydroxamate reveals a dodecameric machinery built by domain-swapped dimers, in agreement with electron microscopy data. A structural comparison with bacterial homologues identifies unifying catalytic features among the poorly understood M18 enzymes. The bound ligands in the active site also reveal the coordination mode of the binuclear zinc centre and a substrate specificity pocket for acidic amino acids. Conclusions: The DNPEP structure provides a molecular framework to understand its catalysis that is mediated by active site loop swapping, a mechanism likely adopted in other M18 and M42 metallopeptidases that form dodecameric complexes as a self-compartmentalization strategy. Small differences in the substrate binding pocket such as shape and positive charges, the latter conferred by a basic lysine residue, further provide the key to distinguishing substrate preference. -

Eelgrass Sediment Microbiome As a Nitrous Oxide Sink in Brackish Lake Akkeshi, Japan
Microbes Environ. Vol. 34, No. 1, 13-22, 2019 https://www.jstage.jst.go.jp/browse/jsme2 doi:10.1264/jsme2.ME18103 Eelgrass Sediment Microbiome as a Nitrous Oxide Sink in Brackish Lake Akkeshi, Japan TATSUNORI NAKAGAWA1*, YUKI TSUCHIYA1, SHINGO UEDA1, MANABU FUKUI2, and REIJI TAKAHASHI1 1College of Bioresource Sciences, Nihon University, 1866 Kameino, Fujisawa, 252–0880, Japan; and 2Institute of Low Temperature Science, Hokkaido University, Kita-19, Nishi-8, Kita-ku, Sapporo, 060–0819, Japan (Received July 16, 2018—Accepted October 22, 2018—Published online December 1, 2018) Nitrous oxide (N2O) is a powerful greenhouse gas; however, limited information is currently available on the microbiomes involved in its sink and source in seagrass meadow sediments. Using laboratory incubations, a quantitative PCR (qPCR) analysis of N2O reductase (nosZ) and ammonia monooxygenase subunit A (amoA) genes, and a metagenome analysis based on the nosZ gene, we investigated the abundance of N2O-reducing microorganisms and ammonia-oxidizing prokaryotes as well as the community compositions of N2O-reducing microorganisms in in situ and cultivated sediments in the non-eelgrass and eelgrass zones of Lake Akkeshi, Japan. Laboratory incubations showed that N2O was reduced by eelgrass sediments and emitted by non-eelgrass sediments. qPCR analyses revealed that the abundance of nosZ gene clade II in both sediments before and after the incubation as higher in the eelgrass zone than in the non-eelgrass zone. In contrast, the abundance of ammonia-oxidizing archaeal amoA genes increased after incubations in the non-eelgrass zone only. Metagenome analyses of nosZ genes revealed that the lineages Dechloromonas-Magnetospirillum-Thiocapsa and Bacteroidetes (Flavobacteriia) within nosZ gene clade II were the main populations in the N2O-reducing microbiome in the in situ sediments of eelgrass zones. -

A Critical Base Pair in K-Turns That Confers Folding Characteristics and Correlates with Biological Function
ARTICLE Received 16 Apr 2014 | Accepted 2 Sep 2014 | Published 29 Oct 2014 DOI: 10.1038/ncomms6127 OPEN A critical base pair in k-turns that confers folding characteristics and correlates with biological function Scott A. McPhee1, Lin Huang1 & David M.J. Lilley1 Kink turns (k-turns) are widespread elements in RNA that mediate tertiary contacts by kinking the helical axis. We have found that the ability of k-turns to undergo ion-induced folding is conferred by a single base pair that follows the conserved AG pairs, that is, the 3b3n position. A Watson–Crick pair leads to an inability to fold in metal ions alone, while 3n ¼ Gor3b¼ C (but not both) permits folding. Crystallographic study reveals two hydrated metal ions coordinated to O6 of G3n and G2n of Kt-7. Removal of either atom impairs Mg2 þ - induced folding in solution. While SAM-I riboswitches have 3b3n sequences that would predispose them to ion-induced folding, U4 snRNA are strongly biased to an inability to such folding. Thus riboswitch sequences allow folding to occur independently of protein binding, while U4 should remain unfolded until bound by protein. The empirical rules deduced for k-turn folding have strong predictive value. 1 Cancer Research UK Nucleic Acid Structure Research Group, MSI/WTB Complex, The University of Dundee, Dow Street, Dundee DD1 5EH, UK. Correspondence and requests for materials should be addressed to D.M.J.L. (email: [email protected]). NATURE COMMUNICATIONS | 5:5127 | DOI: 10.1038/ncomms6127 | www.nature.com/naturecommunications 1 & 2014 Macmillan Publishers Limited. -

Spatiotemporal Dynamics of Marine Bacterial and Archaeal Communities in Surface Waters Off the Northern Antarctic Peninsula
Spatiotemporal dynamics of marine bacterial and archaeal communities in surface waters off the northern Antarctic Peninsula Camila N. Signori, Vivian H. Pellizari, Alex Enrich Prast and Stefan M. Sievert The self-archived postprint version of this journal article is available at Linköping University Institutional Repository (DiVA): http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-149885 N.B.: When citing this work, cite the original publication. Signori, C. N., Pellizari, V. H., Enrich Prast, A., Sievert, S. M., (2018), Spatiotemporal dynamics of marine bacterial and archaeal communities in surface waters off the northern Antarctic Peninsula, Deep-sea research. Part II, Topical studies in oceanography, 149, 150-160. https://doi.org/10.1016/j.dsr2.2017.12.017 Original publication available at: https://doi.org/10.1016/j.dsr2.2017.12.017 Copyright: Elsevier http://www.elsevier.com/ Spatiotemporal dynamics of marine bacterial and archaeal communities in surface waters off the northern Antarctic Peninsula Camila N. Signori1*, Vivian H. Pellizari1, Alex Enrich-Prast2,3, Stefan M. Sievert4* 1 Departamento de Oceanografia Biológica, Instituto Oceanográfico, Universidade de São Paulo (USP). Praça do Oceanográfico, 191. CEP: 05508-900 São Paulo, SP, Brazil. 2 Department of Thematic Studies - Environmental Change, Linköping University. 581 83 Linköping, Sweden 3 Departamento de Botânica, Instituto de Biologia, Universidade Federal do Rio de Janeiro (UFRJ). Av. Carlos Chagas Filho, 373. CEP: 21941-902. Rio de Janeiro, Brazil 4 Biology Department, Woods Hole Oceanographic Institution (WHOI). 266 Woods Hole Road, Woods Hole, MA 02543, United States. *Corresponding authors: Camila Negrão Signori Address: Departamento de Oceanografia Biológica, Instituto Oceanográfico, Universidade de São Paulo, São Paulo, Brazil. -

High Quality Permanent Draft Genome Sequence of Chryseobacterium Bovis DSM 19482T, Isolated from Raw Cow Milk
Lawrence Berkeley National Laboratory Recent Work Title High quality permanent draft genome sequence of Chryseobacterium bovis DSM 19482T, isolated from raw cow milk. Permalink https://escholarship.org/uc/item/4b48v7v8 Journal Standards in genomic sciences, 12(1) ISSN 1944-3277 Authors Laviad-Shitrit, Sivan Göker, Markus Huntemann, Marcel et al. Publication Date 2017 DOI 10.1186/s40793-017-0242-6 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Laviad-Shitrit et al. Standards in Genomic Sciences (2017) 12:31 DOI 10.1186/s40793-017-0242-6 SHORT GENOME REPORT Open Access High quality permanent draft genome sequence of Chryseobacterium bovis DSM 19482T, isolated from raw cow milk Sivan Laviad-Shitrit1, Markus Göker2, Marcel Huntemann3, Alicia Clum3, Manoj Pillay3, Krishnaveni Palaniappan3, Neha Varghese3, Natalia Mikhailova3, Dimitrios Stamatis3, T. B. K. Reddy3, Chris Daum3, Nicole Shapiro3, Victor Markowitz3, Natalia Ivanova3, Tanja Woyke3, Hans-Peter Klenk4, Nikos C. Kyrpides3 and Malka Halpern1,5* Abstract Chryseobacterium bovis DSM 19482T (Hantsis-Zacharov et al., Int J Syst Evol Microbiol 58:1024-1028, 2008) is a Gram-negative, rod shaped, non-motile, facultative anaerobe, chemoorganotroph bacterium. C. bovis is a member of the Flavobacteriaceae, a family within the phylum Bacteroidetes. It was isolated when psychrotolerant bacterial communities in raw milk and their proteolytic and lipolytic traits were studied. Here we describe the features of this organism, together with the draft genome sequence and annotation. The DNA G + C content is 38.19%. The chromosome length is 3,346,045 bp. It encodes 3236 proteins and 105 RNA genes. The C. bovis genome is part of the Genomic Encyclopedia of Type Strains, Phase I: the one thousand microbial genomes study. -

Killing of Gram-Negative Bacteria by Lactoferrin and Lysozyme
Killing of gram-negative bacteria by lactoferrin and lysozyme. R T Ellison 3rd, T J Giehl J Clin Invest. 1991;88(4):1080-1091. https://doi.org/10.1172/JCI115407. Research Article Although lactoferrin has antimicrobial activity, its mechanism of action is not full defined. Recently we have shown that the protein alters the Gram-negative outer membrane. As this membrane protects Gram-negative cells from lysozyme, we have studied whether lactoferrin's membrane effect could enhance the antibacterial activity of lysozyme. We have found that while each protein alone is bacteriostatic, together they can be bactericidal for strains of V. cholerae, S. typhimurium, and E. coli. The bactericidal effect is dose dependent, blocked by iron saturation of lactoferrin, and inhibited by high calcium levels, although lactoferrin does not chelate calcium. Using differing media, the effect of lactoferrin and lysozyme can be partially or completely inhibited; the degree of inhibition correlating with media osmolarity. Transmission electron microscopy shows that E. coli cells exposed to lactoferrin and lysozyme at 40 mOsm become enlarged and hypodense, suggesting killing through osmotic damage. Dialysis chamber studies indicate that bacterial killing requires direct contact with lactoferrin, and work with purified LPS suggests that this relates to direct LPS-binding by the protein. As lactoferrin and lysozyme are present together in high levels in mucosal secretions and neutrophil granules, it is probable that their interaction contributes to host defense. Find the latest version: https://jci.me/115407/pdf Killing of Gram-negative Bacteria by Lactofernn and Lysozyme Richard T. Ellison III*" and Theodore J. Giehl *Medical and tResearch Services, Department of Veterans Affairs Medical Center, and Division ofInfectious Diseases, Department ofMedicine, University ofColorado School ofMedicine, Denver, Colorado 80220 Abstract (4). -

Atypical Parkinsonism–Associated Retromer Mutant Alters Endosomal Sorting of Specific Cargo Proteins
JCB: Report Atypical parkinsonism–associated retromer mutant alters endosomal sorting of specific cargo proteins Kirsty J. McMillan,1 Matthew Gallon,1* Adam P. Jellett,1* Thomas Clairfeuille,3 Frances C. Tilley,1 Ian McGough,1 Chris M. Danson,1 Kate J. Heesom,2 Kevin A. Wilkinson,1 Brett M. Collins,3 and Peter J. Cullen1 1School of Biochemistry and 2Proteomics Facility, School of Biochemistry, University of Bristol, Bristol BS8 1TD, England, UK 3Institute for Molecular Bioscience, The University of Queensland, St. Lucia, Queensland 4072, Australia The retromer complex acts as a scaffold for endosomal protein complexes that sort integral membrane proteins to vari- ous cellular destinations. The retromer complex is a heterotrimer of VPS29, VPS35, and VPS26. Two of these paral- ogues, VPS26A and VPS26B, are expressed in humans. Retromer dysfunction is associated with neurodegenerative disease, and recently, three VPS26A mutations (p.K93E, p.M112V, and p.K297X) were discovered to be associated with atypical parkinsonism. Here, we apply quantitative proteomics to provide a detailed description of the retromer interac- tome. By establishing a comparative proteomic methodology, we identify how this interactome is perturbed in atypical parkinsonism-associated VPS26A mutants. In particular, we describe a selective defect in the association of VPS26A (p.K297X) with the SNX27 cargo adaptor. By showing how a retromer mutant leads to altered endosomal sorting of specific PDZ ligand–containing cargo proteins, we reveal a new mechanism for perturbed endosomal cargo sorting in atypical parkinsonism. Introduction Retromer is a highly conserved heterotrimer of VPS29, homologue (WASH) complex (McGough et al., 2014a; Za- VPS35, and VPS26. -

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants.